Medicinal plants in Europe containing pyrrolizidine alkaloids.
This is the full text of Prof. Dr. E. Röder's article in "Pharmazie" 50 (1995), pages 83-98.
On this site with Prof. Röder's permission. Don't copy this to your site; feel free to link to it instead. The URL is http://www.henriettes-herb.com/articles/PAs.html
This article has German roots, and it shows. It's written by a pharmacist, not an herbalist, and that, too, shows. Be that as it may, it is still the sanest scientific paper on PA-containing plants that I've seen.
1. Introduction
Numerous intoxications in animals and humans caused by the consumption of certain plants were attributed from the middle of this century to compounds of vegetable origin, the pyrrolizidine alkaloids (PAs). In Uzbekistan [1, 2], Afghanistan [3, 4] and India [5-8] up to 6000 people fell victim to mass intoxications. These accidents caused a great sensation.
These intoxications were ascribed to bread cereals contaminated by the seeds of different Heliotropium and Crotalaria species.
Less spectacular but also very harmful were intoxications caused by medicinal plants belonging to the genera Senecio and Crotalaria.
Especially in Jamaica and in other parts of the West Indies [9], in South Africa [10], Central Africa but also in other tropical and subtropical countries [11, 12] characteristic liver diseases such as cirrhosis and primary tumors with a high mortality occurred due to occasional or continued consumption of medicinal plants (bush-teas). These diseases mainly appeared in developing countries because there the consumption of herbs in folk medicine plays a more important role than in highly industrialized countries like in Great Britain or the USA. But also from these countries intoxications caused by confusions or contaminations with PA-containing plants were reported [13-16].
In view of the numerous cases of intoxications and secondary diseases reported from many parts of the world their scientific treatment became a topic of the "United Nations Environmental Program, the International Labor Organization and the World Health Organization" [17].
Although in the eighties in Europe, especially in the Federal Republic of Germany, in Switzerland and Austria the "Green Wave" claimed that medicinal plants may only have a therapeutic effect but no undesired secondary effects, intoxications and casualties were also reported from these countries in connection with PA-containing medicinal plants [18-20]. Due to the hazards that may arise in this context the <German> Federal Health Department (BGA in Berlin) of the Federal Republic of Germany has in order to reduce health risks caused by pharmaceuticals drastically restricted the manufacture and sale of pharmaceuticals containing PAs with a 1,2-unsaturated necine skeleton [21]. Exempt from this restriction are PA-containing pharmaceuticals if a daily administration of 0.1 µg with internal and 10 µg with external application is not exceeded. This special regulation only applies to PA-containing homeopathic pharmaceuticals from a degree of potency of D6 with internal and D4 with external application [22].
2. The Chemistry of Pyrrolizidine Alkaloids.
2.1. Necines
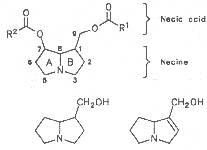
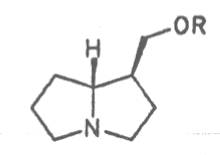
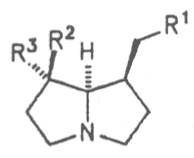
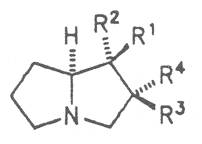
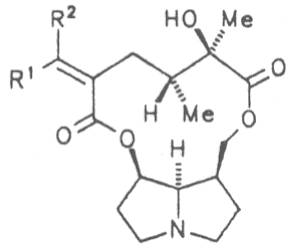
Most medicinal plants discussed in this review article contain PAs of the ester type the basic components of which, called necines, are derived from bicyclic amino alcohols which, in turn, are derived from 1-hydroxypyrrolizidine. The necine can either be saturated or possess a double bond in the 1,2-position (ring B, Fig. 1).
Moreover, they may additionally bear one or two hydroxy groups at C-2, C-6 or C-7 resulting in the formation of stereoisomers. Dashed and thickened (wedges) lines denote a- and b-orientations of bonds, respectively; a meaning orientation away from the observer, b toward the observer. With a few exceptions the bases of most alkaloids belong to the C-8 a series [23]. Figure 2 compiles the necines of the alkaloids found in the medicinal plants discussed in this article.
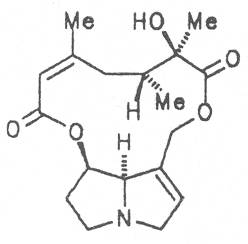
A special role plays otonecine because it is not a genuine bicyclic five-membered ring system but an N-methylated azacyclooctan-4-one system. It may act as a pyrrolizidine ring system due to transannular interactions. The binding between the N atom and the CO group is widered to such an extent that the indicated resonance structures result. The PAs derived from these structures constitute a subgroup of the otonecine alkaloids (OPAs). The corresponding esterification of necines containing a double bond in the 1,2-position results in the formation of the toxic alkaloids (Fig. 1).
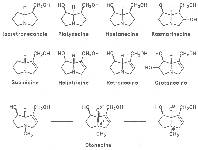
Fig. 2: Necines occurring in the PAs of medicinal plants
2.2. Necic acids
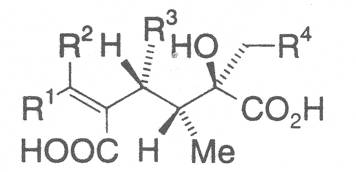
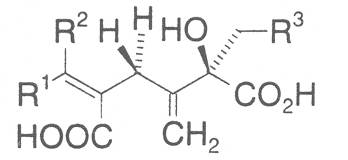
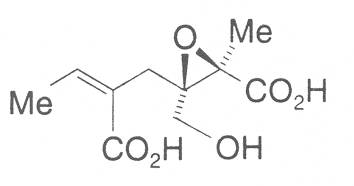
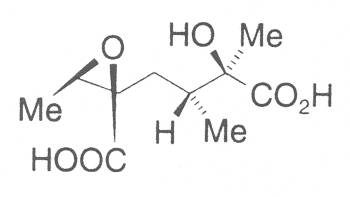
The acids with which the necines are esterified are called necic acids. Apart from acetic acid they possess 5 to 10 C atoms and differ from each other in their structure. They include mono- and dicarboxylic acids with branched carbon chains which are based on simple structural constituents. They bear as substituents hydroxy, methoxy, epoxy, carboxy, acetoxy or other alkoxy groups besides methoxy substituent. Thus numerous structural, stereo- and diastereoisomers may be derived. In Figs. 3-6 are listed the most important mono- and dicarboxylic acids that have been detected in alkaloids so far.
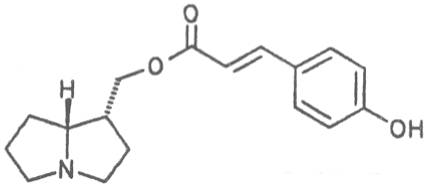
The esterification possibilities are exemplified by several alkaloids. Necines containing one hydroxy group can be esterified with one monocarboxylic acid only as shown in Fig. 7 for amabiline. Necines bearing two hydroxy groups such as 7,9-necinediols can be esterified with a monocarboxylic acid either in the 7- or 9-position as demonstrated by 7-angeloyl respectively 9-angeloylretronecine.
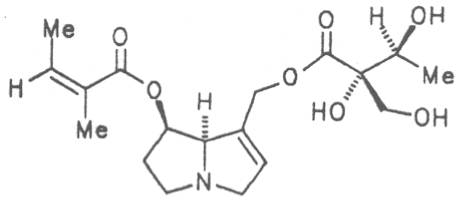
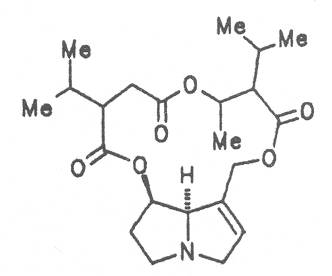
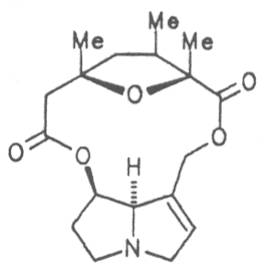
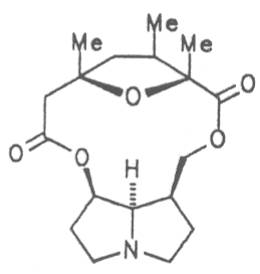
Echimidine is an example of a twofold esterification. With dicarboxylic acids a double esterification takes place exclusively leading to the formation of alkaloids with 11- to 14-membered ring systems. The most widely known PAs are the 11-membered monocrotaline, the 12-membered alkaloids senecionine and senkirkine, the 13-membered doronenine, and the 14-membered parsonsine.
Fig. 3: The most important monocarboxylic acids occurring in PAs
8-C-acids: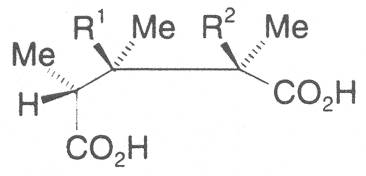 |
|||
| R1 | R2 | ||
| OH | OH | Monocrotalic acid | |
| H | OH | Crotaleschenic acid | |
10-C-acids: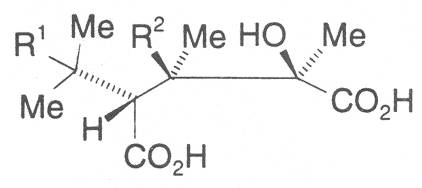 |
|||
| R1 | R2 | ||
| H | H | Incanic acid | |
| H | OH | Trichodesmic acid | |
| OH | OH | Globiferic acid | |
Fig. 4: The most important dicarboxylic acids used for the construction of 11-membered macrocyclic PAs
Fig. 5: The most important dicarboxylic acids used for the construction of 12-membered macrocyclic PAs
Through combination of necines with necic acids an unimaginably large number of alkaloids may be theoretically obtained. In nature ca. 350 alkaloids were found so far and their structures elucidated.
With the exception of about 30 known otonecine alkaloids, which cannot form N-oxides, together with the N-oxides of the other alkaloids more than 640 alkaloids are known [24-29].
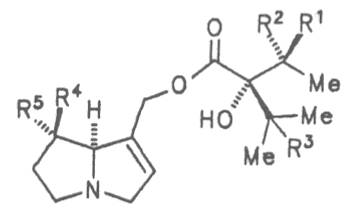
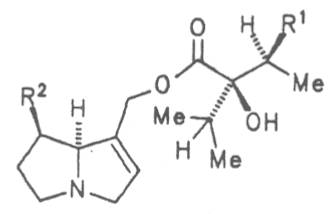
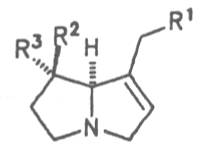
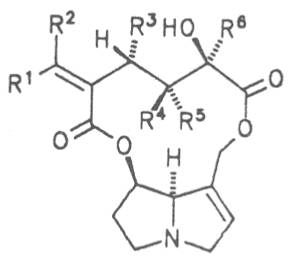
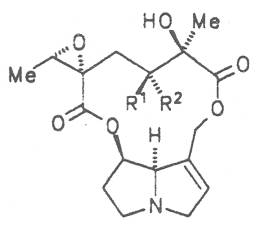
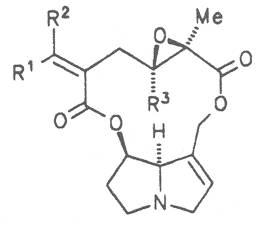
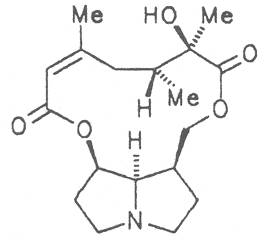
Fig. 8: Structures of pyrrolizidine alkaloids detected in medicinal plants:
| R1 | R2 | R3 | R4 | R5 | R6 | |||
| H | H | CH3 | H | CH3 | CH3 | Senecivernine | 39 | |
| CH3 | H | H | H | CH3 | CH3 | Senecionine | 40 | |
| H | CH3 | H | H | CH3 | CH3 | Integerrimine | 41 | |
| CH3 | H | H | H | CH3 | CH2OH | Retrosine | 42 | |
| H | CH3 | H | H | CH3 | CH2OH | Usaramine | 43 | |
| H | CH2OH | H | H | CH3 | CH3 | 21-Hydroxyintegerrimine | 44 | |
| CH3 | H | H | —CH2— | CH3 | Seneciphylline | 45 | ||
| H | CH3 | H | —CH2— | CH3 | Spartioidine | 46 | ||
| CH3 | H | H | —CH2— | CH2OH | Riddelline | 47 | ||
 |
||||||||
| R1 | R2 | |||||||
| CH3 | H | Platyphylline | 48 | |||||
| H | CH3 | Neoplatyphylline | 49 | |||||
 |
Retroisosenine | 50 | ||||||
 |
Nemorensine | 51 | ||||||
 |
Senkirkine | 52 | ||||||
 |
||||||||
| R=OH | Floridanine | 53 | ||||||
| R = Cl | Doronine | 54 | ||||||
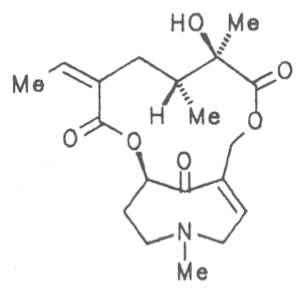
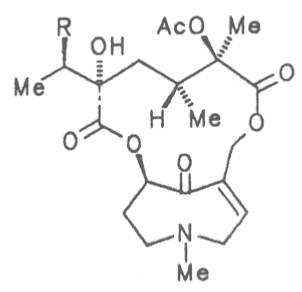
Fig. 8: Structures of pyrrolizidine alkaloids detected in medicinal plants
3. Biosynthesis of pyrrolizidine alkaloids.
The biosynthetic routes were elucidated by a number of investigations performed with 2H-, 3H-, 13C-, 14C-, and 15N-labeled compounds. Both necine bases and necic acids are produced in the amino acid metabolism.
3.1. Biosynthesis of necines
As illustrated in Scheme 1 biosynthesis starts with decarboxylation of the amino acids L-arginine and L-ornithine by the action of arginine and ornithine decarboxylase leading to the formation of putrescine [30-37]. From two putrescine molecules homospermidine is formed. This step, the most important one in the biosynthesis of alkaloids, is catalyzed by the specific enzyme homospermidine synthetase (HS) [38-40]. Homospermidine is cyclized to the corresponding intermediate iminium ion which is reduced with further cyclization to the 1-hydroxymethylpyrrolizidines isoretronecanole and trachelanthamidine. Subsequent hydroxylation and dehydration afford retronecine which is usually a basic constituent of the toxic pyrrolizidine alkaloids. Otonecine, on the other hand, basic component of the otonecine alkaloids, is produced from retronecine, presumably by further hydroxylation and formation of a ketonic group with simultaneous cleavage of the C-N bond and N-methylation. In accordance with these steps is the observation that in cell cultures of Senecio vernalis after a certain period the content of senecionine N-oxide decreases while that of senkirkine, secondary product of otonecine, increases [41-43].
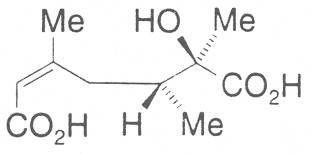
Fig. 6: Dicarboxylic acid used for the construction of 13-membered macrocyclic PAs
|
Senecionine |
|||
Fig. 7 Esterification possibilities of necines with mono- and dicarboxylic acids
Scheme 1: Biosynthesis of necines
3.2. Biosynthesis of necic acids
Necic acids are mainly composed of L-valine, L-leucine, L-isoleucine, and the secondary product of the latter, the L-threonine. In contrast to the necine biosynthesis the synthesis of necic acids follows different routes. In the threonine metabolism monocarboxylic acids with 5 C atoms such as angelic, tiglic and sarracinic acid are generated. Threonine the metabolism of which proceeds via a -ketobutyric acid can interact with pyruvate to furnish isoleucine which may be involved in the formation of necic acids. Isoleucine can be degraded to propionyl-Co A and acetyl-Co A via tiglyl-Co A. The different biosynthetic routes leading to these products are compiled in Scheme 2 [44-61].
Scheme 2: Biosynthesis of monocarboxylic acids
Valine is converted into senecioic, viridifloric and trachelanthic acid via an acyloin reaction with activated acetaldehyde. The formation of the 10 C atom-containing dicarboxylic acids is only effected by subsequent cylization of the open-chain necine monocarboxylic acid diesters. Thus, according to Bourauel, senecionine is produced from diangeloylretronecine in a reaction similar to the Michael addition proceeding via kationic intermediates [62]. The different metabolic pathways thus enable a great variability in the formation of necic acids.
Scheme 3: Biosynthesis of dicarboxylic acids
Biosynthesis takes place in the roots where the alkaloids occur as N-oxides. Being available in easily water-soluble form, they are transported to the aerial parts of the plant and stored there in vacuoles [63, 64].
4. Occurrence of pyrrolizidine alkaloids.
Pyrrolizidine alkaloids are products of the secondary metabolism and mainly occur in representatives of the families Fabaceae, Boraginaceae and Asteraceae. In these families their existence is predominantly restricted to several subtribes and genera as seen from the Table. Only those genera are listed in the species of which pyrrolizidine alkaloids have been detected and which play or have played a certain role as medicinal plants.
In Europe the representatives of Fabaceae are insignificant because they do not occur there. Thus, only medicinal plants belonging to the families Boraginaceae and Asteraceae are important.
Table 1: Occurrence of PAs in different families
| Family | Subtribe | Genus |
| Fabaceae (Leguminosae) | Crotalariaceae | Crotalaria |
| Chromolaena | ||
| Lotononis | ||
| Boraginaceae | With the exception of Pulmonaria officinalis [65, 66] PAs have been detected in all studied genera and species | |
| Asteraceae (Compositae) | Eupatorieae | Ageratum |
| Eupatorium | ||
| Senecioneae | Adenostyles | |
| Brachyglottis | ||
| Cacalia | ||
| Chersodoma | ||
| Cineraria | ||
| Crassocephalum | ||
| Doronicum | ||
| Emilia | ||
| Erechtites | ||
| Farfugium | ||
| Gynura | ||
| Homogyne | ||
| Jacmaia | ||
| Kleinia | ||
| Ligularia | ||
| Nardosmia | ||
| Notonia | ||
| Odontocline | ||
| Packera | ||
| Petasites | ||
| Senecio | ||
| Syneilesis | ||
| Tussilago | ||
| Werneria | ||
4.1. Medicinal plants of the family Boraginaceae containing pyrrolizidine alkaloids.
<numbers in (bold) refer to Fig. 8.>
4.1.1. Alkanna tinctoria (L.) Tausch
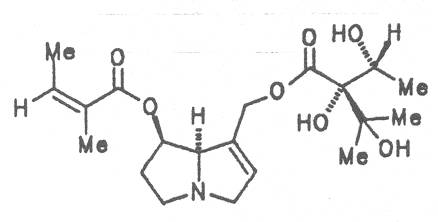
Alcannet (ger. Alkanna, fr. Orcanette tinctoriale, it. Ancusa), grows in South Europe, Turkey and Egypt and is still today cultivated in these countries. From time immemorial the root of this herb is applied internally as antidiarrheal agent and externally in cases of skin diseases. Due to its intensively purple-colored dyestuffs, called alkannins, the bark of the root is also used for staining cosmetics and foods. Three open-chain alkaloids, 7-angeloylretronecine (32), triangularine (35) and dihydroxytriangularine (25), were isolated from the bark of the root in a total concentration of 0.25 to 0.3% [67]. The drug should however no longer be administered internally. Prior to staining of foods, e.g. of lemonades, alkaloids should previously be eliminated.
4.1.2. Anchusa officinalis L.
Common bugloss (ger. Ochsenzunge, fr. Buglosse officinale), is widely distributed in Europe. The aerial parts of the plant are occasionally applied externally with contused injuries, ulcus cruris, sciatica, as compresses with diseases of a joint and of the adjacent bones, internally as a mild expectorant. The total alkaloid content of the dried drug is ca. 0. 12%. Besides the nontoxic alkaloids laburnine (1) and acetyl-laburnine (2) it contains the toxic alkaloids intermedine (4), lycopsamine (5) and 7-acetyllycopsamine (7) [68-70]. Common bugloss should no longer be used as medicinal plant.
4.1.3. Borago officinalis L.
Borage (ger. Boretsch, fr. Bourache, it. Boragine) is a native plant. It is distributed all over Europe from Denmark to Spain and North Africa. It is cultivated in North America. Borage herb and flowers are not only used as salade and spice but also in folk medicine as depuratives, prophylactics against inflammation of the chest and peritoneum, in cases of rheumarthritis, cough and throat diseases as well as phlebitis.
Besides the toxic alkaloids intermedine (4), lycopsamine (5) and their 7-acetyl derivatives (6, 7) these materials contain in very small amounts the slightly toxic amabiline (17), supinine (18) and the nontoxic thesinine (3). The total alkaloid amount is less than 0.001% relative to the dry weight [71, 72]. The <German> Federal Health Administration (BGA) does not regard a use of the claimed fields of applications as justified [73]. On the other hand, there are no objections against an occasional consumption as a spice.
4.1.4. Cynoglossum officinale L.
Common bugloss, common hounds tongue, Beggar's-lice, (ger. Hundszunge, fr. Cynoglosse officinale, it. Cinoglosa) is widely distributed in Europe. In folk medicine the flowering herb and the roots are applied externally in cases of injuries and sprainings, internally as antidiarrheal and a mucolytic agent. Common bugloss contains the alkaloids heliosupine (19), the N-oxide of the latter, 12-acetylheliosupine (20), echinatine (23), and 7-angeloylheliotridine (36) [74, 75]. The alkaloids found by Mankov et al. and Sykulska et al. by paper chromatography (cynoglossophine, viridiflorine, lasiocarpine, heliotrine and platyphylline) could not be confirmed [76-81]. Since the total content of alkaloids varying from 0.7 to 1.5% is very high neither the drug nor preparations there of should be administered. The BGA considers a therapeutic application no longer justified [82].
4.1.5. Heliotropium arborescens L, (syn. H. corymbosum) Ruiz. et Pav.
Garden heliotrope, (ger. Vanille-Heliotrop, Vanille-Sonnenwendkraut, fr. Fleur des Dames, it. Eliotropio) grows in Peru and Ecuador. In Europa it is cultivated as fragrant ornamental plant. In medicine it is also applied in homeopathy in cases of laryngitis and displacement of the uterus. In an older study it was claimed on the basis of a TLC comparison that the drug contained the alkaloid lasiocarpine (21) [83].
A more recent investigation revealed, however, that the plant does not contain lasiocarpine (21) but indicine (28) and 12-acetylindicine (29), respectively the N-oxides of the two compounds in a total concentration of 0.007% in leaves and of 0.01% in roots [84]. Since PA-containing homeopathic preparations may be used for human purposes externally from D4 on and internally from D6 on there are no objections concerning applications of the indicated limiting doses in human medicine [85].
4.1.6. Lithospermum officinale L.
Gromwell, Stone-seed (ger. Steinsame, fr. Grémil officinal, it. Miglio des Sole) is widely distributed in Europe. In folk medicine it is used as antipyretic and gout remedy as well as in cases of urolithic and intestinal complaints. Fresh plant extracts are supposed to display an anticonceptive effect [86]. From the seeds of the gromwell the alkaloids lithosenine (26) and 12-acetyllithosenine (27) could be isolated in a total concentration of 0.003% [87]. The plant or the seed thereof should, if possible, no longer be used.
4.1.7. Myosotis scorpioides L. (syn. M. palustris (L.) Hill)
True forget-me-not, (ger. Vergissmeinnicht, fr. Myosotis des Marais) is a widespread plant in Europe. In folk medicine it is used internally as a sedative and tonic, externally in eye baths. In the dried plant the alkaloids myoscorpine (11), scorpioidine (15), 7-acetylscorpioidine (16), and symphytine (12) were detected in a total content of 0.08% [88, 89]. In any case the application of forget-me-not is considered obsolete.
4.1.8. Symphytum asperum Lepech
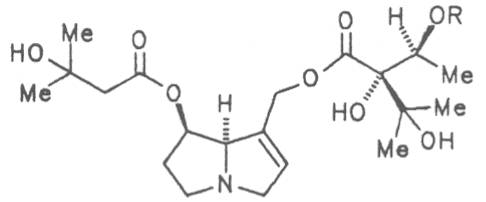
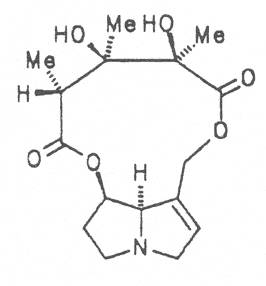
Prickly comfrey, Consound. (ger. Rauher Beinwell, fr. Consoud èpineux), growing in Caucasia is cultivated and used as medicinal and useful plant in the southern parts of Russia and in Central Europe like S. officinale and S. x uplandicum. In the dried leaves the total alkaloid content amounts up to 0.13%, in the dried roots up to 0.14 to 0.37%. In these the alkaloids resp. the N-oxides of intermedine (4) and lycopsamine (5), the 7-acetyl derivatives of the latter two alkaloids (6, 7) symlandine (9), symviridine (10), myoscorpine (11). symphytine (12) as well as the major alkaloid echimidine (13) typical of S. asperum were detected [90, 91]. The presence of the alkaloids asperumine, lasiocarpine, echiumine, makrotomine, and rousorine found in the older studies of Manko et al. by means of paper chromatography could not be confirmed [92-95]. Prickly comfrey should no longer be used for medicinal purposes.
4.1.9. Symphytum caucasicum Bieb.
Caucasian comfrey (ger. Kaukasischer Beinwell, fr. Consoud de Caucase) grows in Caucasia and is used there and in the European part of Russia as folk medicine. In these areas it plays nearly the same important role as S. officinale in Central Europe. In the seventieth it was repeatedly studied by Manko et al. who found a total content of 0.48% of PAs.
Among these the following alkaloids were detected by means of paper and thin-layer chromatography: the N-oxide of echimidine (13), asperumine, echinatine, and lasiocarpine [96-97]. Since the latter three alkaloids have not been found so far in other species of the genus Symphytum further investigations of S. caucasicum are required. Owing to its high alkaloid content an internal intake of this comfrey as a medicinal plant is not recommendable.
4.1.10. Symphytum officinale L. (syn. S. consolida (L.))
Comfrey, Common Comfrey (ger. Beinwell, Wallwurz, fr. Consoude officinale, it. Consolida maggiore), is widespread in Europe. The area of distribution extends from Denmark to Central Russia. Both leaves and roots are used externally in cases of fractures, contused injuries, sprainings, contusions strains, thrombophlebitis, mastitis, hematoma in the form of extracts, ointments, compress pastes, etc., internally as infusions and extracts in cases of gastro-intestinal diseases an respiratory tract diseases. For vegetarians numerous recipe are offered for the preparation of comfrey salade, spinach soufflés, soupes, bread, rolls, and root beverages [98-100].
In dried leaves 0.02 to 0.18 and in the roots 0.25 to 0.29% alkaloids resp. their N-oxides were detected. The alkaloid include intermedine (4), lycopsamine (5), their 7-acetyl derivatives (6, 7), symlandine (9), symviridine (10), myoscorpine (11), and symphytine (12) [101-109]. The presence of the alkaloids (echinatine, heliosupine N-oxide, heliotrine, lasiocarpine, and viridiflorine) isolated and characterized by a Russian and Polish working group by means of paper chromatography could not be confirmed [110-113].
A possible risk associated with the consumption of Symphytum in humans was repeatedly reported [114-117] and in medical literature several cases of intoxication attributed to Symphytum and comfrey are described [118-127]. In animal tests acute toxic effects were detected in rats and goat [128-130].
Rats given for a longer period root drug or a mixture of the alkaloids intermedine and lycopsamine, that are also contained in the comfrey, showed insuloma tumors of the pancreas, liver adenomas, hemangioendothelial sarcomata, and tumors of the bladder.
In the long-term test with the root drug carcinogenicity could be attributed to the main alkaloid symphytine [128, 129]. Moreover, administration of the total alkaloid extract resulted in a mutagenic effect [131, 132]. In view of the risks associated with the alkaloid content neither the leaves nor the root should be used internally. However, under certain conditions there are no objections to an external use provided the skin is intact. In a study on the percutaneous absorption of alkaloids from an alcoholic plant extract that had been applied to the skin of rats 0.08 to 0.41% alkaloid N-oxides wen detected in the urine even after two days [133]. For therapeutic purposes the BGA permits ointments and other preparation with 5 to 20% of dried drug to be applied externally, the daily administered dose being not allowed to contain more than 10 mg of alkaloids including their N-oxides [134]. These requirements are met by the application of species of very low alkaloid content [135].
4.1.11. Symphytum tuberosum L.
Tuberous comfrey (ger. Knoten-Beinwell, fr. Consoud tubereuse), grows in South-East Europe. Its allantoin content is very high; besides, it contains symlandine (9), echimidine (13) and anadoline (14) respectively the N-oxides of these alkaloids in a total concentration of only 0.02% [136-138].
Tuberous comfrey is recommended for medicinal purposes as an alternative to the other comfrey species [138].
4.1.12. Symphytum x uplandicum Nyman (syn. S. peregrinum Ledeb.)
Russian comfrey (ger. Russischer Beinwell, fr. Consoud de Russe), is a hybrid generated from Symphytum officinale L. and S. asperum Lepech. It originates in Caucasia and has become widespread in Central Europe, England, Canada, USA, Australia, New Zealand, Japan, Kenia and South Africa. Under the designation of Russian comfrey it is not only cultivated as medicinal plant but also on a large scale as vegetable, fodder-and fertilizer plant. Roots and leaves contain intermedine (4), lycopsamine (5), the 7-acetyl derivatives (6, 7) of these alkaloids, uplandicine (8), symlandine (9), symviridine (10), myoscorpine (11), symphytine (12), the major alkaloid echimidine (13), and the N-oxides of these compounds in different concentrations. A total alkaloid content of ca. 0.2% was found in the dried aerial parts [90, 91, 139, 140]. Russian comfrey should no longer be used for medicinal purposes.
4.2. Medical plants of the family Asteraceae containing pyrrolizidine alkaloids.
<numbers in (bold) refer to Fig. 8.>
4.2.1. Eupatorium cannabinum L.
Hemp agrimony, Thoroughwort (ger. Kunigundenkraut, Wasserdost, fr. Eupatoire chanvrine, it. Canape aquatica), is a widespread plant in Europe which preferentially grows on humid soils and brook edges. The aerial, flowering parts are used as immunostimulating agent in cases of influenza infections, as a remedy against obstipation and for decreasing the cholesterol level as well as a diuretic. In these parts of the plant the unsaturated alkaloids intermedine (4), lycopsamine (5), amabiline (17), supinine (18), rinderine (22), and echinatine (23) were detected in different concentrations [141-146]. A continued use, e.g. for the purpose of decreasing the cholesterol level, should be renounced.
4.2.2. Adenostyles alliariae (Gouan) Kern
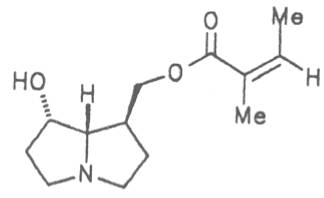
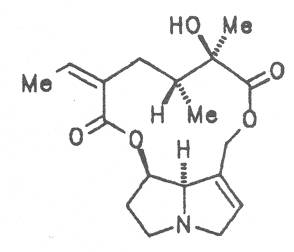
Grey adenostyl (ger. Grauer Alpendost, fr. Adénostyle alliaire), is widespread in the countries of the Alps where it grows preferentially in the region of the timber-line. Until the beginning of this century it was used by the inhabitants of the Alps as a pectoral tea and as such was relatively important. It contains the alkaloids senecionine (40), seneciphylline (45) and spartioidine (46), the total alkaloid content being ca. 0.02% and thus relatively high [147-150]. Recently, a heavy liver disease (venoocclusive disease) in an infant caused by erroneous ingestion of grey adenostyl instead of coltsfoot was reported [151].
4.2.3. Emilia sonchifolia (L.) DC.
Emilia herb (ger. Emilienkraut), is a medicinal plant widespread in the south of China. It is used there in folk medicine as antipyretic, as a remedy against influenza and grippal dysentery and analgetic. Following Tyrolian physician Leonhard Hohenegg, this herb is praised as a particularly effective remedy against fever, influenza, cough, and bronchitis in lay medicine [152]. Emilia herb contains senkirkine (52) and doronine (54) in a total concentration of up to 0.2% [153]. Application of the amounts of this herb recommended by Hohenegg would inevitably result in intoxication.
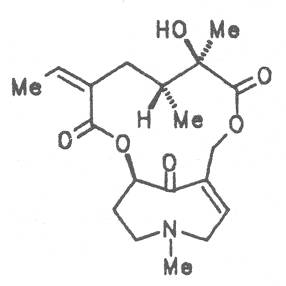
4.2.4. Petasites hybridus (L.) PH Gaertn., B. Mey & Scherb. (syn. P. officinalis Moench)
Pestilence-wort, Butter bur, Colts food (ger. Pestwurz, Grosser Huflattich, fr. Pétasite vulgaire, it. Cavo laccio, Petastite, Tussilagine maggiore), occurs in Central Europe. Its distribution extends from Denmark up to North and West Asia. It preferentially grows on river banks and brooks. The leaves and roots of this plant and preparations thereof are applied in cases of nervous and painful spasmodic stages of different genesis in the gastro-intestinal tract, liver, gallbladder and pancreas diseases, with headache, respiratory tract disease, and for the promotion of sleep. Besides the alkaloids senecionine (40), integerrimine (41), senkirkine (52), the dried root drug contains trances of petasitenine (57), neopetasitenine (58), the nontoxic alkaloids neoplatyphylline (49), isotussilagine (68), and tussilagine (70) in a total amount of 1 to 100 mg per kilogram, the leaf drug contains less than one tenth of this amount [154-156]. According to the BGA the root-stock may be applied as therapeutic agent in the treatment of acute convulsive pain in the region of the abdomen. The daily dose administered must not contain more than 1 mg of the alkaloids or alkaloid N-oxides and the duration of application is limited to four to six weeks per year [134].
4.2.5. Petasites spurius (Retz) RCHB
Spurious pestilence-wort (ger. Filziger Pestwurz, fr. Pétasite bâtard, Petit Taconnet), grows on the sandy river-banks of the Elbe, Saale, Havel, Oder, and Spree. In Europe its distribution area extends from Denmark, South Sweden, South Poland to Central Russia. In the Baltic Sea countries mentioned the rhizome was or is still used internally as a cough remedy. In addition to the toxic senkirkine (52) with a concentration of ca. 0.007% also the nontoxic alkaloids farfugine (31), isotussilagine (68), isotussilaginine (69), and tussilagine (70) were detected in this plant [157]. An internal intake cannot be recommended.
4.2.6. Senecio aureus L.
Golden ragwort, Squaw weed, Life root, Stinking Willie (ger. Gold-Kreuzkraut, fr. Seneçon d'or, it. Senecione aureono) is widely distributed in North America and Canada where it grows on humid river-bank meadows. Already the Red Indians cultivated this ragwort as medicinal plant in many ways. Today it is still used by the aborigines as a remedy against injuries, internally as diaphoretic, diuretic and emmenagogue. The Red Indian wives today ingest high doses of this drug both for acceleration of the the course of labor and abortion. In Europe, especially in Germany, it is administered in homeopathy as mother tincture and as dilutions down to D3 in the case of hemorrhage of various kinds of genesis in gynecology. The drug contains the alkaloids floridanine (53), otosenine (55) and florosenine (56) in a total concentration of 0.02% [158, 159]. The presence of senecionine could not be confirmed [160]. According to the regulations of the BGA golden ragwort is allowed to be applied internally for therapeutic purposes in humans only in concentrations from D6 on.
4.2.7. Senecio bicolor (Wild.) Tod. ssp. cineraria (syn. S. cineraria DC., syn. Cineraria maritima L.)
Dusty miller, cineraria ragwort, silver groundsel (ger. Cineraria-Kreuzkraut, Aschenkraut, fr. Seneçon cineraire) grows in the Mediterranean areas and is cultivated in Europe as ornamental plant. Extracts of the flowering herb are administered as eye drops in the treatment of corneal clouding, cataracts and conjunctivitis especially in the southern EU countries (Portugal). In the flowering plants senecionine (40), retrorsine (42), seneciphylline (45), otosenine (55), jaconine (60), and jacobine (61) were detected in a total concentration of 0.9% [161-167]. According to the regulations of the BGA in the Federal Republic of Germany eye drops are allowed to be applied externally as a homeopathic pharmaceutical with a degree of potency of D4 [22].
4.2.8. Senecio doronicum L.
Doronic ragwort (ger. Gamswurz-Kreuzkraut, fr. Seneçon Doronic, it. Cardonella) grows on lime soils in the Alps. Infusions of the flower-head were used by the inhabitants of the Alps as a remedy against asthma. The flowering plants contain the alkaloids doronenine (66) and bulgarsenine (67) [168, 169]. Administration of the doronic ragwort as a remedy against asthma is regarded as obsolete.
4.2.9. Senecio jacobaea L.
Tansy ragwort, European ragwort, (ger. Jakobs-Kreuzkraut, fr. Seneçon Jacobèe, it. Jacobea) is widespread in Europe. Until the beginning of this century it was used as antispasmodic, emmenagogue and in gynecology in cases of functional amenorrhea. Tansy ragwort contains a large number of PAs. The total alkaloid content amounts to 0.2 to 0.3% of the dry weight of the flowering plants. The following alkaloids were isolated from the plant: senecivernine (39), senecionine (40), integerrimine (41), retrorsine (42), usaramine (43), 21-hydroxyintegerrimine (44), seneciphylline (45), spartioidine (46), riddelline (47), jacoline (59), jaconine (60), jacobine (61), jacozine (62), (Z)-erucifoline (63), (E)-erucifoline (64), and acetylerucifoline (65) [170-190]. In short- and long-term tests performed on mice and rats the intake of both the powdered drug and extracts caused acute toxic and carcinogenic effects [191-193]. In the Ames test ingestion of an alkaloid extract gave rise to a mutagenic effect [194]. Of course, this medicinal plant should not be used.
4.2.10. Senecio nemorensis L. ssp. fuchsii C. Gmel. (S. nemorensis L. ssp. fuchsii Celak.), and S. nemorensis ssp. nemorensis (S. nemorensis ssp. jacquinianus (RCHB.) Celak.)
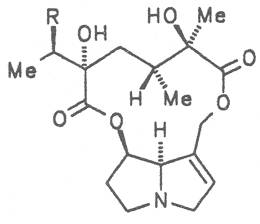
Both subspecies Fuchsii ragwort (ger. Fuchs-Kreuzkraut, fr. Seneçon de Fuchs), and Nemorensis ragwort, (ger. Hain-Kreuzkraut, fr. Seneçon de Jaquin), are closely related to each other, thus allowing formation of hybrids. The distribution area of fuchsii ragwort extends from Central Europe via the northern parts of the Balkan to Caucasia. It frequently grows in forests of the montaneous regions and moreover up to an altitude of 2000 m. Nemorensis ragwort occurs throughout the European-Siberian areas up to a maximum height of 1500 m. The two herbs are applied as hemostyptic agents both in dentistry and gynecology. Recently they have also been recommended and applied as hypoglycemic agents in cases of hypertension and diabetes. Fuchsii and nemorensis ragwort contain besides the open-chain PAs of the unsaturated retronecine and saturated platynecine type such as 7- and 9-angeloylretronecine (32, 33), 7-senecioylretronecine (34), triangularine (35), fuchsisenecionine (37), and sarracine (38) also macrocyclic 12- and 13-membered, saturated and unsaturated alkaloids. These include platyphylline (48), nemorensine (51), bulgarsenine (67), senecionine (40), retroisosenine (50), doronenine (66), and the N-oxides of these in very different concentrations [195-202]. The medicinal effect is ascribed to the nontoxic, saturated alkaloids fuchsisenecionine and bulgarsenine which represent the largest proportion of the alkaloids. In a toxicologic long-term test on rats ingestion of a commercial dried drug containing besides the nontoxic major alkaloid fuchsisenecionine in a concentration of 0.37% the toxic senecionine in a concentration of 0.007% caused a dose-dependent hepatotoxic and carcinogenic effect. This effect is attributed to senecionine [203]. An alkaloid mixture of the same concentration displayed weak mutagenic effects [203, 204]. As hemostyptic agent only extracts of Senecio nemorensis ssp. fuchsii should be administered because in these extracts the portion of toxic alkaloids is much lower than that in S. nemorensis ssp. nemorensis. The portion of toxic alkaloids of the retronecine type such as senecionine, retroisosenine, and doronenine should therefore be less than 1 mg. Diabetics who frequently had taken ragwort tea for several years in order to support their therapy should not use such extracts because of the health risks associated with their ingestion. The BGA generally considers a medicinal application of fuchsii ragwort and nemorensis ragwort no longer justified [134].
4.2.11. Senecio vulgaris L.
Common ragwort, Common groundsel, Grindsel (ger. Gewöhnliches Kreuzkraut, fr. Seneçon vulgaire, it. Cardoncello), is widespread in the plains and mountains of Europe. In folk medicine it played a certain role as emmenagogue and in cases of functional amenorrhoea. Besides, it was applied similarly as Senecio jacobaea. Like the latter it contains an unusually large number of alkaloids up to a total content of 0.16% dry weight of the aerial whole drug. These alkaloids include senecionine (40), integerrimine (41), retrosine (42), usamarine (43), seneciphylline (45), spartoidine (46), riddelline (47), and the corresponding N-oxides [205-219]. But also in this case preparations of this ragwort should neither be recommended nor administered.
4.2.12. Tussilago farfara L.
Coltsfoot, Horsefoot (ger. Huflattich. fr. Pas d'âne, Tussilage, it. Farfaro) is an important medicinal plant not only in Europe and Asia but also in the USA and Canada. It either grows in the latter two countries or has been imported. For medicinal purposes flower buds, flowers, leaves, and roots are used. From time immemorial coltsfoot has been employed in cases of colds, asthma, influenza, gastro-enteritis, diarrhea, for metabolic stimulation, blood purification and externally for the treatment of wounds. Depending on its origin, the dry drug contains different amounts of senkirkine (52) ranging from 0.1 to 150 ppm, in some cases also 0.1 to 10 ppm of senecionine (40), besides the nontoxic alkaloids isotussilagine (68), isotussilaginine (69), tussilagine (70), and tussilaginine (71), usually in a total amount of <2 ppm [220-228]. Owing to the known hepatotoxicity and hepatocarcinogenicity of senkirkine and senecionine a Japanese working group performed a feeding experiment on rats for nearly two years. At nonphysiologic high dosage of dried flower buds hemangiosarcomas, hepatocellular adenomas and carcinomas could be detected [229].
In 1988, intoxication of an infant resulting in death was ascribed to coltsfoot [20]. However, from the description of the case and the analysis of the tea mixture it was concluded that ingestion of coltsfoot was not the factor causing death. The tea mixture contained in addition to coltsfoot leaves also leaves and roots of pestilence-wort [230-232]. The BGA permits only leaves and tea mixtures the toxic PA content of which does not exceed 10 mg. With extracts and fresh plant-pressed juice the limiting value may be 1 mg. The period of application is confined to maximally six weeks a year [134].
4.3. Honey containing pyrrolizidine alkaloids
<numbers in (bold red) refer to Fig. 8.>
Depending on the origin of the nectar collected by the bees, honey also contain PAs. In honey originating from Senecio jacobaea the alkaloids senecionine (40), seneciphylline (45), jacoline (59), jaconine (60), jacobine (61), and jacozine (62) were detected in a total concentration of 0.3 to 3.2 mg [233]. From the honey of Senecio vernalis the alkaloids senecivernine (39), senecionine (40) and senkirkine (52) were isolated in a total concentration of ca. 0.5-1.0 mg [234]. In honey from Echium plantagineum besides echimidine (13) as major component the alkaloids echiumine (30), uplandicine (8) as well as intermedine (4), lycopsamine (5), and the acetyl derivative (6, 7) of the latter two compounds were found in a total concentration of 0.27 to 0.95 mg [235]. Macrocyclic PAs, mainly seneciphylline (45), could be detected in the concentration range of 30-70 mg/kg in a honey sample from the Alpine foothills region of Switzerland [236]. Measurable concentrations of PAs must always be reckoned with in the case of large-area monocultures of PA-containing plants. In England, Scotland and Ireland honey from tansy ragwort is known and notorious for its strong aroma and bitter taste [237]. This honey obviously also contains alkaloids. However, since honey is of minor importance as a staple food an intoxication may hardly occur.
5. Toxicity of pyrrolizidine alkaloids.
Alkaloids derived from 1-hydroxymethyl-l,2-dehydropyrrolizidine and esterified with at least one branched C5 carboxylic acid display a toxic, carcinogenic and mutagenic effect. Nearly 100 of the PAs known so far possess such a structure. Enhancement of the effect occurs if a further hydroxy group is introduced into the 7-position and also esterified, especially if the necinediol is esterified with a dicarboxylic acid to afford a macrocyclic compound. Alkaloid N-oxides, on principle, display the same toxicity. Since they are, in contrast to the bases, extremely water-soluble they are subject to different pharmacokinetics. After oral administration the alkaloids or their N-oxides are resorbed in the intestine, the N-oxides being previously reduced by the reductases of the bacterial flora.
Part of the alkaloids is cleaved into necines and necic acids by the nonspecific esterases of the blood. Necines are nontoxic and are excreted as conjugates via the kidneys and urine. The main proportion of the alkaloids on the other hand is transported into the liver where metabolic conversions induce reactions known as "intoxication reactions" <poisonings>.
5.1. Acute and Chronic Toxicity
In liver cells PAs give rise to the following changes:
- 10- to 30- fold enlargement of the liver cells (megalocytosis)
- enlargement of the liver nuclei with increasing nuclear chromatin
- disturbances of the liver cell metabolism with considerable functional losses
- occurrence of irregular mitoses with simultaneous inhibition of mitosis due to DNA blocking
- cytoclases
- fatty degeneration.
These reactions are induced by a single intake of 10 to 20 mg of an alkaloid or alkaloid mixture.
If cytoclasis comprises larger regions of liver parenchyma death results.
Lower amounts of alkaloids (less than 10 µg) and longer disposition periods give rise to further damages:
- proliferation of biliary tract epithelials
- inflammatory infiltrates
- congestive and centrilobular necrosis of Venae hepaticae (VOD)
- cirrhosis
- generation of carcinomas.
This necrosis has been introduced in the medical literature as Venoocclusive Disease (VOD) and is regarded as specific PA intoxication <poisoning> (seneciosis). It is practically identical with the clinical picture of the Budd-Chiari syndrome. The clinical symptoms usually occur suddenly and comprise:
- colicky pains in epigastrium
- vomiting and diarrhea
- ascites formation within several days
- enlargement and induration of the liver within a few weeks.
In serious cases the following symptoms are additionally observed:
- vasomotoric states of collapse
- hematemesis
- bleeding diarrhea.
The seriousness of the intoxication <poisoning> is not only dependent on the dosis administered but also on endogenic (age, sex) and exogenic (alcohol, drug, living conditions) factors. Children are obviously more sensitive to PA intoxications <poisonings> [238-279]. The Kwashiorkhor disease frequently observed with children in Central Africa is also related to a damage of liver cells by PAs, the liver having lost its ability to synthesize endogenic proteins [280, 281].
Besides these effects on the liver, severe toxic pulmonary damages with pulmonary-arterial hypertension and subsequent right ventricular failure were observed. When the metabolities can no longer be trapped in the liver cells they are transported via the blood into the pulmonary arterioles where they cause damage to the endothelial vessels. In the capillaries the latter are stimulated to proliferation causing a mediahypertrophy in the arterioles and thus a pressure increase in the pulmonary circulation and acute right ventricular failure similar to the classical cor pulmonale [282-286].
The toxicity of several PAs could be directly corroborated by means of the "yeast test" performed with Saccaromyces cerevisiae [287, 288].
5.2. Carcinogenicity of pyrrolizidine alkaloids
A subtoxic intake (less than 1 mg) of PAs over longer periods resulted in the following gradually occurring changes of organs:
- megalocytosis
- VOD
- fatty degeneration
- proliferation of the biliary tract epithelials
- liver cirrhosis
- nodular hyperplasia
- adenomas or carcinomas.
The frequent occurrence of primary liver tumors in the natives of Central Africa and South Africa is ascribed to the consumption of traditional medicinal plants of the genera Crotalaria, Cynoglossum, Heliotropium and Senecio [289-292]. In numerous experiments on animals with plants, with the extracts of the latter or with the alkaloids occurring in the aforementioned plant genera or in their species the clinical picture could be confirmed [293-311].
5.3. Mutagenicity and genotoxicity of pyrrolizidine alkaloids
The mutagenicity of PAs has already been extensively studied. In this review article only those studies referring to the medical plants and the alkaloids contained in the latter are described. The plant extracts or the alkaloid mixtures of Senecio jacobaea, Senecio nemorensis ssp. fuchsii, Senecio fuchsii, and Symphytum officinale investigated in different test systems all exhibited a mutagenic effect [131, 132, 194, 203, 204, 312-314]. The alkaloids having a relation to the discussed medicinal plants also possess mutagenic properties. These alkaloids belong to the plant genera Senecio and Petasites (senecionine, integerrimine, retrosine and N-oxides, seneciphylline, jacobine, senkirkine), Eupatorium (supinine) and Symphytum (intermedine, lycopsamine, the N-acetyl derivatives of these, symphytine, echimidine). The following test systems were used: Escherichia coli, Salmonella typhimurium (Ames), Aspergillus nidulans, Vicia faba, Allium cepa, Drosophila melanogaster, leucocytes from marsupials, hepatic cells from rats and mice, liver cells from Chinese hamsters, mice and cattle as well as human lymphocytes. However, the various test systems used afforded different results. Although it was found that the studied alkaloids exhibit mutagenic properties the potency of the mutagenicity of the individual alkaloids could not be ascertained [315-339]. This was only realized by an extensive investigation of Frei et al. who made use of the wing spot test of Drosophila melanogaster [340]. 16 alkaloids were tested under the same conditions, thus allowing comparative studies and even a quantitative assessment of the genotoxicity to be made for the first time. The following order of decreasing mutagenicity of the alkaloids was established:
senkirkine > monocrotaline > seneciphylline > senecionine > 7-acetyl intermedine > heliotrine > retrorsine > 7-acetyllycopsamine > symphytine > jacoline > symlandine > intermedine > indicine > lycopsamine > indicin N-oxide > supinine.
The series starts with the alkaloid with the highest mutagenicity, the senkirkine. The activity decreases via three powers of ten up to supinine that displays no activity.
Hence, the macrocyclic alkaloids senkirkine and senecionine possess the highest activity. Retrorsine which may be regarded as 12-hydroxymethyl derivative of senecionine exhibits a fivehold weaker mutagenicity. Jacoline which may also be considered as a hydroxy derivative of senecionine likewise exhibits a weak effect. Obviously, increasing hydroxylation of necic acids decreases the mutagenic effect. Among the open-chain diesters 7-acetylintermedine and 7-acetyllycopsamine display a five to ten times weaker activity than the macrocyclic compounds, while the acetyl esters possess a two to three times higher activity than e.g. the alkaloids symphytine and symlandine. The antitumor therapeutic agent indicine N-oxide exhibits only a very weak mutagenic effect. Obviously, also in this case the general principle applies that the mutagenic activity decreases with increasing hydroxylation. By means of this very extensive study the mutagenicity of other alkaloids, which have not been investigated so far, may be assessed.
5.4. Teratogenicity of pyrrolizidine alkaloids
<numbers in (bold) refer to Fig. 8.>
The teratogenic effect of PAs was demonstrated by a single intraperitoneal injection of heliotrine (24) into pregnant rats. At concentrations of 50 to 200 mg alkaloid/kg body weight teratogenic changes were observed. Doses above 200 mg/kg resulted in uterine death and degradation of the fetuses [341]. Similar results were obtained with heliotrine and its metabolite dehydroheliotrine, the latter exhibiting however a teratogenicity which 2.5 times as high as that of the initial alkaloid [342]. The teratogenic properties of heliotrine were also demonstrated by experiments on larvae of fruit flies, Drosophila melanogaster [343].
5.5. Metabolism
5.5.1. Metabolism of 1,2-unsaturated pyrrolizidine alkaloids
Metabolic reactions occur in the liver. A portion of the alkaloids, which, as is well-known, are esters are hydrolyzed by nonspecific esterases. The produced necines are supposed to be excreted renally. Alkaloids of this kind are relatively nontoxic because the necines do not produce toxic metabolites [344-346]. However, if the carboxylic acids contain branched chains hydrolysis is strongly impaired by sterical hindrance. If these difficultly hydrolyzable alkaloids contain as a basic constituent a necine of the supinidine, heliotridine, retronecine, or crotanecine type they are converted into the corresponding N-oxides by microsomal oxygenases. These N-oxides undergo rearrangement and elimination of water to afford the corresponding dehydropyrrolizidine alkaloids. Moreover, hydroxylation of the carbon atoms adjacent to the nitrogen atom (C-3 and C-8) is also supposed to occur, leading to the very unstable 3- and 4-hydroxypyrrolizidine alkaloids. Elimination of water from these alkaloids followed by rearrangement yields the corresponding dehydropyrrolizidine alkaloids [347-3531. As shown in Fig. 9 these alkaloids possess an allyl structure causing an increase in reactivity.
Fig. 9: Allyl structure of dehydropyrrolizidine alkaloids
Also alkaloids of the otonecine type differing from the other PAs by a methyl group at the nitrogen atom and a "quasi" ketonic function at C-8 were degraded similarly. After cationic elimination of the methyl group the nor-N-otonecine alkaloids rearranges to the 8-hydroxy derivative and the latter to the corresponding dehydropyrrolizidine alkaloid.
Dehydropyrrolizidines representing (A)pyrrolidine (B)pyrrole derivatives are highly reactive and are the virtually active species of the PAs.
In the presence of nucleophilic systems (Nu-) the diesters undergo either a simple or a double bimolecular nucleophilic substitution (SN2 reaction). In parallel occurring reactions a hydrolysis leading to the formation of dehydronecines (dehydroretronecine, dehydroheliotridine) may also take place. These compounds are similarly reactive, i.e. their toxicity and carcinogenicity resemble those of dehydroalkaloids.
Scheme 4 delineates the reaction sequence of a twofold bimolecular nueleophilic substitution [354-365].
Scheme 4: Metabolism of 1,2-unsaturated PAs
According to Mattocks et al. the reaction of the macrocyclic alkaloids starts in the 7-position [366]. MNDO (Modified Neglect of Differential Overlaps) calculations, a standard method for determining charges and energy contributions on van-der-Waals-surfaces, especially in the case of heteroatom-containing organic molecules, reveal that locations of scission contain higher partial charges than the other molecular regions. In the case of dehydrosenecionine these locations are at -0.341 for the 9-H2CO and at -0.328 for the 7-HCO group [367].
Fig. 10: Computer diagram with van der Waals surfaces exemplified by dehydrosenecionine
Hydroxy, mercapto or amino groups of enzymes, globulins, hemoglobin but also of purine and pyrimidine bases or their nucleosides may function as nucleophiles. Hence, DNS and/or RNS may also undergo alkylations. Already simple alkylation affords an adduct that causes a lasting change of a DNS and/or RNS strand. A double alkylation may give rise to irreversible cross linking of both strands as shown in Fig. 11. If no repair process occurs a carcinogenic reaction may be definitly predicted [368-387].
Recently, a further metabolic reaction has been assumed. According to Segall et al. the alkaloid, e.g. senecionine, is supposed to be degraded to (E)-4-hydroxy-2-hexenal as demonstrated by the reaction Scheme 5 [388].
Scheme 5: Degradation of senecionine to 4-hydroxy-2-hexenal
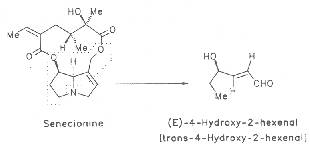
Aldehydes with a double bond in the 2,3-position display liver toxic properties.
Fig. 11: Cross linking of DNS strands by dehydropyrrolizidine
5.5.2. Metabolism of 1,2-saturated pyrrolizidine alkaloids
Saturated pyrrolizidines and their necines are nontoxic. According to Mattocks et al. the alkaloids platyphylline and rosmarine undergo enzymatic hydrolytic cleavage at C-7 with simultaneous formation of a pyrrole unit of ring B as shown in Scheme 6.
Scheme 6: Metabolism of 1,2 saturated PAs
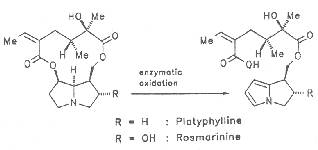
In contrast to dehydro(A)pyrroles the nitrogen atom of which exists in a very unstable conjugated state and may thus readily react with nucleophiles, 1,2-saturated pyrrolizidines are considerably more stable and not susceptible to reactions with nucleophiles [389, 390].
6. Outlook
After it had been demonstrated that test systems containing some alkaloids or alkaloid N-oxides exhibited an antitumor effect several attempts were made to use PAs in medicinal treatment of carcinosis [391-393]. Since indicine itself does not show mutagenic properties its N-oxide (28) was successfully applied in clinical studies on infantile leukemia. Unfortunately, liver damages like liver necrosis and liver cirrhosis were observed as side reactions so that a further therapeutic utilization was no longer justified [394-404].
In the Pharmacopoea Sinica of 1978 the alkaloid monocrotaline is described. It was used for the treatment of dermatomas. On account of its highly toxic and carcinogenic side effects it is not mentioned in the new edition of 1985 and also no longer used.
The saturated alkaloid platyphylline (48) displays spasmolytic and antiasthmatic properties and was included in the Pharmacopoe of the former UDSSR <Soviet Union> (1980). Compared to other spasmolytics and antiasthmatics it exhibits no particular effect so that it has gained no importance.
The use of medicinal plants represents a considerable risk of health. Because of the liberalization of the international world trade an increasing number of medicinal plants are offered as "miracle drugs" and imported to Europe without control as the example of the Emilia herb demonstrates [152, 153]. The medicinal plants of the genera resp. the species of the latter compiled in Table 1 should not be used on any account because they contain toxic PAs which may cause damage to health.
References.
(not spellchecked).
- 1 Dubrovinskii. S. B.: J. Sov. Prot. Health 6, 17 (1946)
- 2 Khanin. M. N.: Arch. Pathol. USSR 1, 42 (1948)
- 3 Tandon. B. N.: Tandon, H. D.: Mattocks, A. R.: Ind. J. Med. Res. 68. 84 (1978)
- 4 Tandon. B. N.; Tandon, H. D.; Mattocks, A. R.: Am. J. Gastroenterol. 70, 607 (1978)
- 5 Tandon. R. K.: Tandon. B. N.; Tandon, H. D.: Bhatia, M. L.: Bhargava, S.: Lal. P.: Arora, R. R.: Gut 17, 849 (1976)
- 6 Tandon. B. N.: Tandon, H. D.: Tandon, R. K.; Narendranathan. M.: Joshi. Y. K.: Lancet 2. 271 (1976)
- 7 Krishnamachari. K. A. V. R.: Bhat, R. V.: Krishnamurthy. D.. Krishnasmamy, K.: Nagarajan, V.: Ind. J. Med. Res. 65, 672 (1977)
- 8 Tandon. H. D.: Tandon. B. N.; Tandon, R.; Nayak, N. C.: Ind. J. Med. Res. 65. 679 (1977)
- 9 Stuart, K. L.: Bras. G.: Quart. J. Med. New Ser. 26, 291 (1957)
- 10 Selzer. G.: Parker. R. G. F.: Chir, M. B.: Am. J. Pathol. 21 885 (1951)
- 11 Schoental. R.: J. Trop. Ped. 2. 208 (1957)
- 12 Culvenor. C. C, J.: in Smith, R. L.: Bababunmi. E. A. (eds.): Toxicology in the Tropics. p. 124. Taylor & Francis Ltd.. London 1980
- 13 McGee. J. O'D.~ Patrick, R. S.: Wood. C. B.; Blumgart. L. H.: J. Clin. Pathol. 29. 788 (1976)
- 14 Huxtable. R. J.: Gen. Pharmacol. 10, 159 (1979)
- 15 Huxtable. R. L: Persp. Biol. Med. 24, 1 (1980)
- 16 Huxtable. R. L: Trends Pharmacol. Sci. 1, 299 (1980)
- 17 Anon.: Ens ironmental Health Criteria 80, Pyrrolizidine Alkaloids. WHO. Geneva 1988
- 18 Huxtable. R. J.: in Cheeke. P. R. (ed.): Toxicants of Plant Origin Vol. 1, p. 41. CRC Press, Inc. Boca Raton, Florida 1989
- 19 Margalith. D.: Heraief. E.; Schindler. A. M.: Birchler. R.: Mosimann. F.; Aladjem. D.: Gonvers. J. J.: J. Hepatol. Suppl. S280/204 (1985)
- 20 Roulet. M.: Laurini. R.; Rivier, L.~ Calame, A.: J. Pediatr. 112. 433 (1988)
- 21 BGA: Abwehr von Arzneimittelrisiken, Stufe 11, Pyrrolizidinalkaloidhaltige Humanarzneimittel~ Az.: GV 7-7251-01-25365, 10. August 1988 cit. Pharm. Ztg. 135. 1-532 (1990)
- 22 BGA: Abwehr von Arzneirnittelrisiken, Stufe 11, BGA, BAnz Nr. 111 S. 4805 v. 17. 06. 1992 cit. Pharm. Ztg. 137, 2088 (1992) and Dtsch. Apoth. Ztg. 132. 1406 (1992)
- 23 Culvenor. C. C. L: Bot. Not. 131. 473 (1978)
- 24 Bull. L. B.: Culvenor. C. C. J.: Dick, A. T.: The Pyrrolizidine Alkaloids, North Holland, Amsterdam 1968
- 25 Smith. L. W.: Culvenor. C. C. L: J. Nat. Prod. 44, 129 (198 1)
- 26 Robins. D. L: Fortschr. Chem. Organ. Naturst. 41, 115 (198-1)
- 27 Roeder. E.: Pharm. Uns. Zeit 13. 33 (1984)
- 28 Mattocks. A. R.: Chemistry and Toxicology of Pyrrolizidine Alkaloids, Academic Press. London, New York, Sydney 1986
- 29 Rizk. A. F. M. (ed.): Naturally Occuring Pyrrolizidine Alkaloids. CRC Press. Boca Raton, Arm Arbor, Boston 1991
- 30 Robins. D. L: Chem. Soc. Rev. 18, 375 (1989)
- 31 Khan, H. A.: Robins. D. J.: J. Chem. Soc., Perkin Trans. 1. 101 (1985)
- 32 Reed. R. L.; Reed. B. M_ Buffier, D. R.: Planta Med. 472 (1985)
- 33 Khan, H. A.: Robins. D. J.: J. Chem. Soc., Perkin Trans. 1. 819 (1985)
- 34 Kelly, A. H.: Robins. D. J.: J. Chem. Soc., Chem. Commun. '29 (1988)
- 35 Kunec, E. K.; Robins, D. J~: J. Chem. Soc., Chem. Commun, 250 (1986)
- 36 Kunec, E. K.; Robins, D. J.: J. Chern. Soc., Perkin Trans. 1, 1437 (1989)
- 37 Robins, D. L: Experientia 47, 1118 (1991) -
- 38 Boettcher, F.; Adolph, R. D.; Hartmann, T.: Phytochemistry 32, 679 (1993)
- 39 Graser, G.; Boetteher, F.; Sander, H.; Hartmann, T.: 41` Arm. Congr. Plant Res. Duesseldorf 31. 08. 1993, P 95
- 40 Boetteher. F.; Ober, D.; Hartmann, T.: 41` Arm. Congr. Plant Res. Duesseldorf 31. 08. 1993, P 96
- 41 Kelly~ H. A.; Kunec, E. K_ Rodgers, M.; Robins, D. L: J. Chem. Res. Synop. 358 (1989)
- 42 Freer. 1. K. A.; Matheson, J. R.; Rodgers, M.; Robins, D. L: J. Chern. Res. Synop. 46 (1991)
- 43 Toppel. G.; Witte, L.; Riebeschl, B.; v. Borstel, K.; Hartmann, T.: Plant Cell. Rep. 6. 466 (1987)
- 44 Hughes, C.. Warren. F. L.: J. Chem. Soc. 34 (1962)
- 45 Crout, D. H. G.; Benn, M. H.; Imaseki, Fl., Geissman, T. A.: Phytochemistry 5. 1 (1966)
- 46 Crout. D. H. G.: J. Chem. Soc. (C) 1968 (1966)
- 47 Crout. D. H. G.: J. Chem. Soc. (C) 1233 (1967)
- 48 Crout. D. H. G. z Davies, N. M.; Smith, E. H.; Whitehouse, D.: J. Chem. Soc.. Chem. Commun. 635 (1970)
- 49 Crout. D. H. G.: Davies, N. M.; Smith, E. El.; Whitehouse, D.: J. Chem. Soc.. Perkin Trans. 1. 671 (1972)
- 50 Davies. N. M.. Crout. D. H. G.: J. Chem. Soc., Perkin 1, 2079 (1974)
- 51 O'Donovan, D. G.: Long, D. L: Proc. R. Ir. Acad. Sect. B 75, 465 (1975): C. A. 84. 28094s (1976)
- 52 Crout. H. G.; Whitehouse, D.: J. Chem. Soc., Perkin L 544 (1977)
- 53 Bale, N. M.; Caltill. R.: Davies, N. M.; Mitchell, M. B.; Smith. E. H.; Crout, D. H. G.: J. Chem. Soc., Perkin 1, 101 (1978)
- 54 Rao. P. G.: Zutshi. U.; Soni. A.; Atal, C. K.: Planta Med. 35. 279 (1979)
- 55 MeGaw, B. A.~ Woolley, J. G.: Phytochemistry 18, 1647 (1979)
- 56 Cahill. R.: Crout. D. H. G.~ Mitchell, M. B.; Mueller, U. S.: J. Chem. Soc., Chem. Commun. 419 (1980)
- 57 Cahill. R.: Crout. D. H. G.: Gregorio, M. V. M.; Mitchell, M. B.: Mueller, U. S.: J. Chem. Soc.. Perkin Trans. 1, 173 (1983)
- 58 Robins. D. L; Bale. N. M.; Crout, D. H. G.: J. Chem. Soc., Perkin Trans. 1, 2082 (1974)
- 59 Des lin. J. A.: Robins. D. J.: J. Chem. Soc., Perkin Trans. L 1329 (1984)
- 60 Denholm. A. A.; Robins, D. L: J. Chem. Soc., Chem. Commun. 19 (1991)
- 61 Rycroft, D. S.: Stirling, 1. R.; Robins. D. J.: Magn. Res. Chem. 30, S42 (199-1)
- 62 Bourauel. T.: Ph. D. Thesis Borm 1993
- 63 Hartmann. T.: Toppel, G.: Phytochemistry 26, 1639 (1987)
- 64 Elirnke. A.: v. Borstel, K.~ Hartmann, T.: Planta 176, 83 (1988)
- 65 Roeder. E.: Britz-Kirstgen, R.: Unpublished results (1984)
- 66 Luethy. J.: Brauchfi, L; Zweifel, U.; Schmid. P.: Schlatter, C.: Pharm. Acta HeR. ~9, 141 (1984)
- 67 Roeder. E.. Wiedenfeld, H.: Schraut, R.: Phytochemistry 23. 2125 (1984)
- 68 Pedersen. E.: Arch. Pharm. Chemi. Sci. Ed. j. 55 (1975): C. A. 83. 175446r (1975)
- 69 Broch-Due. A. L: Aasen, A. L: Acta Chem. Scand. B34, 75 (1980)
- 70 Hendriks. H.~ Bruins, A. P.; Huizing, H. L: Biorned. Environ. Mass. Spectrom. 17. 129 (1988)
- 71 Larson. K. M.; Roby. M. R.: Stermitz, F. R.: J. Nat. Prod. 47, 747 (1984)
- 72 Dodson. C. D.: Stermitz, F. R.: J. Nat. Prod. 49, 727 (1986)
- 73 BGA, Bundesanzeiger 127. S. 4548, v. 12. 07. 1991
- 74 Pedersen, E.: Dan. Tidsskr. Farm. 44, 287 (1970): C. A. 74. 72780e (1971)
- 75 Resch, J. F_ Meinwald. J.: Phytochemistry 21, 2430 (1982)
- 76 Manko, 1. V.: Borisyuk, Y. G.: Ukrain. Khim. Zhur. 23, 362 (1957): C. A. 52. 2187i (1958)
- 77 Manko. 1. V.: Ukrain. Khim. Zhur. 25,627 (1959): C. A. 54, 1-2494c (1960)
- 78 Manko. 1. V.: Farm. Zh. Kiev 19, 22 (1964): C. A. 64, 4125b (1966)
- 79 Sykulska. Z.: Acta Pol. Pharm. 18, 335 (1961): C. A. 56, 8839i (1962)
- 80 Sykulska. Z.: Acta Pol. Pharm. 19, 183 (1961): C. A. 59, 2876e (1963)
- 81 Jerzmanoss ska. Z.: Sykulska, Z.: Diss. Pharm. 16, 71 (1964): C. A. 61, 16438b (1964)
- 82 I3GA, Bundesanzeiger 43, S. 1071, v. 02. 03. 1989
- 83 Carcamo. V. NI.: Bol. Soc. Quim. Peru 27, 161 (1961): C. A. 61, 15032b (1961)
- 84 Roeder. E.: Bourauel, T.: Kersten, R.: Unpublished results, 1994
- 85 BGA: Az.: GV 77251-01-25356/1 (1988)
- 86 Winterhoff. H.~ Gumbinger. H. G.: Dtsch. Apoth. Ztg. IX 2667 (1990)
- 87 Krenn. L.: Wiedenfeld. H.; Roeder, E.: Phytochemistry 35. 275 (1994)
- 88 Resch. J. F.; Meinwald, J.: Tetrahedron Lett. 22, 3159 (198 1)
- 89 Resch, J. F.: Rosberger. D. F.; Meinwald, L; Appling, J. W.: J. Nat. Prod. 45, 358 (1982)
- 90 Roeder. E.: Netiberger, V.: Dtsch. Apoth. Ztg. 128, 1991 (1988)
- 91 Roeder, E.; Bourauel, T.; Neuberger, V.: Phytochemistry 31, 4041 (1992)
- 92 Manko. 1. V. ~ Koto~skii, B. K.~ Stolyarets, V. L: Tr. Leningr. Khim. Farm. Inst. 21. 137 (1967): C. A. 69, 93671g (1968)
- 93 Manko. 1. V.; Korotkova. M. P.; Shes,tsova, N. M.: Rast. Resur. 5, 508 (1969): C. A. 72. 87175u (1970)
- 94 Manko. 1. V.; Kotos,skii, B. K.; Denisov, Y. G.: Rast. Resur. 6, 582 (1970): C. A. 47. 84023y (1971)
- 95 Manko, 1. V.: Kotovskii, B. K.: Zh. Obshch. Khim. 40,2519 (1970): C. A. 75, 1243s 11971)
- 96 Manko. 1. V.: Melkumova, Z. V.; Malysheva, V. F.: Rast. Resur. 8, 538 (1972): C. A. 78, 82085d (1973)
- 97 Melleurnova, Z. V.; Telezlienetskaya, M. V.; Yunusov, S. Y.; Manko, 1. V.: KItim. Prir. Soedin. 478 (1974): C. A. 82, 54177z (1975)
- 98 Hills, L. D.: Comfrey: Past, Present and Future. Faber & Faber, London 1976
- 99 Schloss, L.: Comfrey, Wiedergeburt einer Heilpfianze, Selbstverlag Comfrey Vertrieb GmbH Bergen, Chiemgau 1979
- 100 Anon.: Comfrey, was ist das? Selbstverlag Abtei Fulda 1980
- 101 Furuya, T.; Araki, K.: Chem. Pharm. Bull. 16, 2512 (1968)
- 102 Furuya. T.; Hikichi, M.: Phytochemistry 10, 2217 (1971)
- 103 Pedersen, E: Arch. Pharm. Chem. Sci. Ed. 3, 55 (1975): C. A. 83, 175446r (1975)
- 104 Resch, J. F.; Rosberger, D. E; Meinwald, J.; Appling, J. W.: J. Nat. Prod. 45, 358 (1982)
- 105 Stengl, P.; Wiedenfeld, H_ Roeder, E: Dtsch. Apoth. Ztg. 122, 851 (1982)
- 106 Roeder. E.; Wiedenfeld, H.; Stengl, P.: Arch. Pharm. 315, 87 (1982)
- 107 Mackay. M. F.; Sadek, M.; Culvenor, C. C. 1: Acta Cryst. C39, 785 (1,983)
- 108 Huizing, H. J.; Roeder, E; Wiedenfeld, H.: Arch. Pharm. 318, 476 (1985)
- 109 Huizing, H. J.; Ph.D. Thesis, Groningen 1985
- 110 Makarova, G. V.; Zaraiska, K. N.; Borisyuk, Y. G.: Farm. Zh. (Kiev) 21, 41 (1966): C. A. 66, 49229h (1967)
- 111 Manko, 1. V.: Kotovskii, B. K.; Denisov, Y. G.: Rast. Resur. 6, 409 (1970): C. A. 74, 61608d (197 1)
- 112 Manko. 1. V.; Melkumova, Z. V.; Malysheva, V. E: Rast. Resur. 8, 538 (1972): C. A. 78, 82085d (1973)
- 113 Debska. W.: Owczarka, A.; Madalinska, R.: Herba Pol. 26, 47 (1980): C. A. 93. 225694z (1980)
- 114 Mattocks. A. R.: Lancet 2, 1136 (1980)
- 115 Huxtable, R. J.: Trends Pharmacol. Sci. 1, 299 (1980)
- 116 Roitman. J. N.: Lancet 1. 944 (1981)
- 117 Anderson, C.: Lancet 1, 1424 (1981)
- 118 H uxtable. R. J.: Luethy, J.; Zweifel, U.: New Engl. J. Med. 315, 1095 (1986)
- 119 Ridger. P. M.: Olikurna, S.; McDermott, W. V.; Trey, C.: Huxtable, R. 1: Gastroenterology 88, 1050 (1985)
- 120 Vollmer. J. J.: Steiner. N. C.; Larsen, G. Y.; Muirhead. K. W Molyneux, R. L: J. Chem. Educ. 64. 1027 (1987)
- 121 a Weston. C. F. M_ Cooper, B. T.; Davies, J. W Levine, D. F.: Br. Med. J. 295, 183 (1987)
121 b Abbott, P. J.: Med. J. Aust. 149, 678 (1988) - 122a Ridker. P. M.: MeDermott. W. V.: Lancet 1, 657 (1989)
122b Bach. X: Thung, S. N.: Schaffner, E: Am. J. Med. 87, 97 (1989) - 123 Yeong. M. L.: Swinburn, B.: Kennedy. M.; Nicholson, G.: J. Gastroent. Hep. 5. 211 (1990)
- 124 McDermott. W. V.: Ridker. P. M.: Arch. Surg. 125, 525 (1990)
- 125 Winship. K. A.: Adverse Drug React. Toxicol. Rev. 10, 47 (1991)
- 126 Garret. B. L: Cheeke. P. R.; Miranda, C. L.; Goeger, D. E; Bultler, D. R.: Tox. Lett. 10, 183 (1982)
- 127 Yeong. M. L.: Clark, S. P.; Waring, J. M., Wilson. R. D.; Wakefield. S. J.: Pathology 23. 35 (1991)
- 128 Hirono. 1.~ Mori, H.; Haga. M.: J. Nati. Cancer Inst. 61, 865 (1978)
- 129 Hirono. I.: Haga, M.: Fujii, M.; Matsuura, S.: Matsubara. N.: Nakayama. M.: Furuya. T.; Hikichi, W Takanashi, H.; Uchida, E; Hosaka. S.: Ueno. I.: J. Nati. Cancer Inst. 63, 469 (1979)
- 130 Roeder, E.: Ncuberger. V.; Greuel. E: Vogel, J.: Unpublished results (1987)
- 131 Furmanowa. M.: Guzewska. J.; Beldowska, B.: J. Appl. Tox. 3. 127 (1983)
- 132 Belminger. C.; Abel, G.; Roeder. E.; Neuberger, V.z Goeggelmann. W.: Planta Med. 55. 518 (1989)
- 133 Brauchli. L~ Luethy. L; Zweifel, U.~ Schlatter. C.: Experientia 38, 1085 (1982)
- 134 BGA. Bundesanzeiger 138, S. 3866 v. 27. 07. 1990
- 135 Muetterlein, R.; Arnold. C. G.: Pharm. Ztg. Wiss. 13816, 119 (1993)
- 136 Ulubelen, A.: Doganca, S.: Tetrahedron Lett. 2583 (1970)
- 137 Ulubelen. A.: Oecal, E: Phytochemistry 16, 499 (1977)
- 138a Gray. A. I.: Saoir, E. X; Waterman, P. G.: J. Pharm. Pharmacol. 35,13P. Suppl. (1983)
138b 13handari. P.: Gray, A. L: J. Pharm. Pharmacol. 37, 50P (1985) - 139 Culvenor. C. C. L; Edgar, J. A.; Frahn, J. L.; Smith. L. W.: Aust. J. Chem. 33, 1105 (1980)
- 140 Culvenor. C. C. J.; Clarke, M.; Edgar, J. A.; Frahn, J. L.~ Jago, M. V.; Peterson. J. E; Smith, L. W.: Experientia 36, 377 (1980)
- 141 Pedersen. E: Phytochemistry 14, 2086 (1975)
- 142 Huizing. H. L: De Boer, F.: Hendriks, H.; Bairaadjsing, W.; Bruins, A. P.: Biomed. Environ. Mass. Spectrom. 13, 293 (1986)
- 143 Hendriks. H.; Balraadjsing, W.; Huizing, H. J.; Bruins, A. P.: Planta Med. 53, 456 (1987).
- 144 Schmid, P.; Luethy, L; Zweifel, U.; Bettschart. A.z Schlatter, C.: Mitt. Gebiete Lebensm. Hyg. 78~ 208 (1987)
- 145 Pagani, E: Boll. Chim. Farm. 129, 281 (1990): C. A. 115, 228434y (1991)
- 146 Huelsmeyer, P.~ Witte, L.; Hartmann, T.: 40th Arm. Congr. Med. Plant Res., Trieste 1. - 5. 09. 1992, 23/P3
- 147 Pimenov, M. G.~.Yakhontova, L. D.; Pakalne, D.; Sapunova, L. A.: Rast. Resur. 72 (1975): C. A. 82, 152218h (1975)
- 148 Yakhomova. L. D- Pimenov, M. G.; Sapunova. L. A.: Khim. Prir. Soedin. 122 (1976): C. A. 85, 59575z (1976)
- 149 Wiedenfeld, H.; Knoch, E; Roeder, E; Appel, R.: Arch. Pharm. 317, 97 (1984)
- 150 Roeder. E; Plassmeier, C.: Sci. Pharm. 59, 301 (1991)
- 151 Sperl, W.~ Stuppner, H.; Gassner, I.; Judmaier, W.; Dietze. 0.; Vogel, W.: Eur. J. Pediatr. in press (1994)
- 152 Hoehue, A.: ,Heiltees, die Wunder wirken. Die Geheirmezepte des Tiroler Arztes Dr. med. Leonhard Hochenegg", p. 27, 67, 79, Ariston-Verlag, Genf 1986
- 153 Roeder, E; Cheng, D. L.: Planta Med. 484 (1986)
- 154 Luethy, L; Zweifel, U.; Schmidt, P.; Schlatter, C.: Pharm. Acta Helv. 58, 98 (1983)
- 155 Mauz, C.; Candrian, U.; Luethy, L; Schlatter, C.; Sery, V.; Kulm, G.; Kade, E: Pharm. Acta Helv. 60, 256 (1985)
- 156 Roeder, E; Wiedenfeld, H.; Schraut, R.: Unpublished results (1983)
- 157 Roeder, E.; Eckert, A.; Bourauel, T.: Pharmazie 48, 953 (1993)
- 158 Resch, J. E; Goldstein, S. A.; Meinwald, J.: Planta Med. 48, 255 (1983)
- 159 Roeder, E; Hoenig, A.; Wiedenfeld, H.: Planta Med. 49, 57 (1983)
- 160 Manske, R. H. E: Can. J. Res. 17, 1 (1939)
- 161 Barger, G.; Blackie, J. J.: J. Chem. Soc. 584 (1937)
- 162 Adams, R.; Govindachari, T. R.: J. Am. Chem. Soc. 71, 1953 (1949)
- 163 Alekseev, V. S.; Bilyuga, T. G.; Taldikin. 0. E.: Farm. Zh. (Kiev) 17, 42 (1962): C. A. 57, 7384h (1962)
- 164 Alekseev, V. S.; Bankovskii, A. L: Farm. Zh. (Kiev) 20, 49 (1965): C. A. 64, 9997g (1966)
- 165 Habib, A. A. M.: Planta Med. 26, 279 (1974)
- 166 Klasek, A.; Mnatsakanyan, V. A.; Santavy, F.: Collect. Czech. Chem. Commun. 40, 2524 (1975)
- 167 Dvorackova, S.; Santavy, E; Klasek, A.: Acta Univ. Palacki Olomuc. Fac. Med. 86, 33 (1978): C. A. 92, 37777j (1980)
- 168 Roeder, E; Wiedenfeld, H.; Frisse, M.: Phytochemistry 19, 1275 (1980)
- 169 Kirfel, A.; Will, G.: Wiedenfeld, H.; Roeder. E: Cryst. Struct. Commun. 9, 353 (1980)
- 170 Manske, R. H. E: Can. J. Res. 5, 651 (1931)
- 171 Barger, G.; Blackie, J. L: J. Chem. Soc. 584 (1937)
- 172 Blackie, J. L: Pharm. J. 138, 102 (1937)
- 173 Bradbury, R. B.; Culvenor, C. C. L: Aust. J. Chem. 7, 378 (1954)
- 174 Bradbury, R. B.~ Culvenor. C. C. L: Chem. Ind. (London) 1921 (1954)
- 175 Adams, R.; Gianturco, M. L: J. Am. Chem. Soc. 78, 398 (1956)
- 176 Bradbury, R. B.: Aust. J. Chern. 9, 521 (1956)
- 177 Bradbury. R. B.: Willis, 3. B.: Aust. J. Chem. 9, 258 (1956)
- 178 Bradbury, R. B.; Mosbauer, S.: Chern. Ind. (London) 1236 (1956)
- 179 Bradbury, R. B.; Masamune, S.: J. Am. Chem. Soc. 81, 5201 (1959)
- 180 Geisman, T. A.: Aust. J. Chem. 12, 247 (1959)
- 181 Culvenor, C. C. J.: Aust. J. Chem. 17, 233 (1964)
- 182 Alekseev. V. S.; Bankovskii, A. J.: Farm. Zh. (Kiev) 20, 49 (1965): C. A. 64, 9997g (1966)
- 183 Akramov. S. T.: Shadmanov, Z; Samatov, A.; Yunusov, S. Y.: Khim. Prir. Soedin. 4, 258 (1968): C. A. 70, 44831 ss. (1969)
- 184 Ferry, S.: Arm. Pharm. Fr. 30, 145 (1972)
- 185 Ferry, S.: Brazier, J. L.: Arm. Pharm. Fr. 34. 133 (1976)
- 186 Segall, H. 1: Tox. Lett. 1. 279 (1978)
- 187 Cheeke, P. R. (ed.); Deinzer. M. L.; Thomson. P. A.: Griflin, D. A.; Burgett. D. M.: Proc. Symp. Pyrrozidine (Seneciol Alkaloids: pp. 11 - 169. Corvallis, Oregon. State University, 23-24. 02. 1979
- 188 Dimenna. G. R: Krick. T. P.: Segall. H. J.: J. Chromatogr. 192,474 (1980)
- 189 Pieters, L. A. C.: van Zoelen. A. M.: Vrieling. K.: V1ietinck, A. J.: Magn. Res. Chem. 27, 745 (1989)
- 190 Witte, L_ Ernst. L.: Adam, H.; Hartmann. T.: Phytochemistry 31, 559 (1992)
- 191 Cook. J. W.: Duffy, E: Schoental, R.: Br. J. Cancer 4, 405 (1950)
- 192 Schoental. R.; Head, M. A.; Peacock. P. R.: Br. J. Cancer 8, 458 (1954)
- 193 Hooper, P. T.: J. Pathol. 113, 227 (1974)
- 194 White, R. D.; Krumperman, P. H.; Cheeke. P. R.; Buffier, D. R.: Tox. Lett. 15, 25 (1983)
- 195 Klasek, A.: Sedmera. P.; Boeva. A.: Santavy. F.: Collect. Czech. Chem. Commun. 38. 2504 (1973)
- 196 Lemp, G.: Planta Med. 24, 386 (1973)
- 197 Nguyen. T. N.: Sedmera. P.; Klasek, A.: Boeva. A.: Dryanovska, L.: Dolejs. L.; Santavy, E: Collect. Czech. Chem. Commun. 14, 2952 (1976)
- 198 Roeder. E; Wiedenfeld. H.: Phytochemistry 16, 1462 (1977)
- 199 Wiedenfeld, E; Roeder, Phytochemistry 18, 1083 (1979)
- 200 Klasek. A.: Sedmera, P.; Vokoun, L; Boeva. A.: Dvorackova, S.; Santavy, E: Collect. Czech. Chem. Commun.
- 201 Wiedenfeld, H.; Hendriks, H.; Bruins, A. P.; Roeder, E.: Sci. Pharm. 57, 97 (1989)
- 202 Gottlieb, R.; Dtsch. Apoth. Ztg. 130, 285 (1990)
- 203 Habs, H.; Habs, M.; Marquardt, H.; Roeder, E.; Schmaehl, D.; Wiedenfeld, H.: Drug Res. 32, 144 (1982)
- 204 Pool, B. L.: Toxicology 24, 351 (1982)
- 205 Manske, R. F. .L.: Can. J. Res. B13, 6 (1936)
- 206 Barger, G.; Blackie, J. J.; J. Chem. Soc. 743 (1936)
- 207 Konovalova, R. A.; Orekhov, A. P.;; Bull. Soc. Chim. Fr. 4, 1285 (1937): C. A. 31, 74367 (1937)
- 208 Adams, R.; Govindachari, T.R.: J. Am. Chem. Soc. 71, 1956 (1949)
- 209 Shun, T.; Koluch, J.; Santavy, F.: Collect. Czech. Chem. Commun. 25, 934 (1960)
- 210 Gharbo, S.A.; Habib, A. A. M.: Lloydia 32, 506 (1969)
- 211 Ferry, S.: Ann. Pharm. Fr. 30, 145 (1972)
- 212 Ferry, S.; Brazier, J. L.: Ann. Pharm. Fr. 34, 133 (1976)
- 213 Qualls, jr., C. W.; Segall, H. J.; J. Chromatogr. 150, 202 (1978)
- 214 Segall, H.J.: J. Liq. Chromatogr. 2, 1319 (1979)
- 215 Pieters, L. A. C.; Vlietinck, A. J.: Fresenius Z. Anal. Chem. 321, 355 (1985)
- 216 Pieters, L. A. C.; Vlietinck, A. J.: Magn. Res. Chem. 25, 8 (1987)
- 217 Pieters, L. A. C.; Vlietinck, A. L: Planta Med. 54, 178 (1988)
- 218 Hartmann, T.; Efunke, A.; Eilert, U.; v. Borstel, K.; Theuring, C.: Planta 177, 98 (1989)
- 219 Ingolfsdottir, K.; Hylands, 1: Acta Pharm. Nord. 2, 343 (1990)
- 220 Botbaev. A. L; Pleklianova, N. V.: Organ. Khimiya i Puti RazAtiya Khim. Proiz-v'v Kirgizii 106 (1976): C. A. 87, 180671x (1977)
- 221 Culvenor, C. C. J.; Edgar, J. A.; Smith, L. W.; Hirono, J. H.: Aust. J. Chem. 29, 229 (1976)
- 222 Borka. L.: Onshuus, L: Medd. Norsk, Farm. Selsk. 41, 165 (1979): C. A. 92, 3235n (1980)
- 223 Luethy. L; Zweifel, U.; Schlatter, C.; Benn, M. H.: Mitt. Geb. Lebensm. Hyg. 71. 73 (1980)
- 224 Rosber2er, D. F.; Resch, J. F.; Meinwald, J.: Mitt. Geb. Lebensm. Hyg. 72, 432 (1981)
- 225 Roeder. E.; Wiedenfeld, H.; Jost, E.J.: Planta Med. 43, 99 (198 1)
- 226 Wiedenfeld, H.; Roeder, E.; Kirfel, A.; Will, G.: Arch. Pharm. 316,367 (198 1)
- 227 Roeder. E.; Jost, E.-J.: Unpublished results (1984)
- 228 Steinbach, R. A.; Miething, H.: Pharm. Ztg. 134, 25 (1989)
- 229 Hirono. L; Mori, H.; Culvenor, C. C. J.: Gann 67, 125 (1976)
- 230 Roeder. E.: Dtsch. Apoth. Ztg. 128, 2321 (1988)
- 231 Spang. R.: J. Pediatr. 115, 1025 (1989)
- 232 Glornbitza, K.-W.; Roeder. E.: Unpublished results (1988)
- 233 Deirizer. M. L.; Thomson, P. A.; Burgett, D. M.; Isaacson, D. L.: Science 195, 497 (1977)
- 234 Roeder. E.; Pastewka, U.; Wiedenfeld, H.: Unpublished results (1981)
- 235 Culvenor, C. C. J.; Edgar, J. A.; Smith, L. W.: J. Agric. Food Chem. 29, 958 (1981)
- 236 Luethy. L: Schmid. P.; Zweifel, U.; Schlatter, C.: Unpublished results (1983)
- 237 Maurizio. A.: Grall, L: Das Trachtpfianzenbuch, p. 171, Ehrenwirth Verlag, Munich. 1978
- 238 Wilmot. F. C.: Robertson, G. W.: Lancet 2, 848 (1920)
- 239 Hashem. M.: J. Egypt. Med. Assoc. 22, 319 (1939)
- 240 Prabhu. M. B.: Ind. J. Pediatr. 7, 121 (1940)
- 241 Ismaelos. N.: Klin. Med. (Moscow) 26.23 (1948)
- 242 Roves. K.: Caribb. Med. J. 10, 16 (1948)
- 243 SeIzer. G.: Parker. R. G. F.: Am. J. Pathol. 27, 885 (195 1)
- 244 Savvina. K. L: Arch. Pathol. 14, 65 (1952)
- 245 Bras, G.: JellifTe. D. B.; Stuart, K. L.: Arch. Pathol. 57. 285 (1954)
- 246 Jelliffe. D. B.z Bras. G.: Stuart, K. L.: Paediatr. 14, 334 (1954)
- 247 Stuart. K. L.: Bras, G.: Br. Med. J. 2, 348 (1955)
- 248 Bras. G.: Watler. D. C.: West Ind. Med. J. 4~ 201 (1955)
- 249 Stuart. K. L.: Bras. G.: West Ind. Med. J. 5, 33 (1956)
- 250 Bras. G.: Hi11 K. R.: Lancet 2. 161 (1956)
- 251 Stuart. K. L.; Bras. G.: Q. J. Med. 26, 291 (1957)
- 252 Jelliffe. D. B.: Bras. G.: Mukherjec. K. L: Arch. Dis. Child. 32,369 (1957)
- 253 Stein. H.: Br. Med. J. 1496 (1957)
- 254 Hill. K. R.: Proc. R. Soc. Med. 53, 281 (1960)
- 255 Bras.G.: Brooks.S. E. H.; Watler, D. C.J. Pathol. Bacteriol.82.503 (1961)
- 256 Stein. H.: Isaacson. C.: Br. Med. J. 372 (1962)
- 215 7 Stirlinj. G. A.; Bras. G.~ Urquhart, A. E.: Arch. Dis. Child. 37.535 (1962)
- 258 Gupta. P. S.: Gupta. G. D.: Sharma, M. L.: Brit. Med. J. L 1184 (1963)
- 259 Davidson. C. S.: New Engl. J. Med. 268. 1072 (1963)
- 260 Safouli. Shchata, A. H.: J. Pediatr. 67, 415 (1965)
- 261 B ' raginski. B. M.; Bobokhadzaev, 1. Y.: Sov. Med. 28. 57 (1965)
- 262 Jago. M. V.: Am. J. Pathol. 56, 405 (1969)
- 263 Najak. N. C.: Sagreya. K.: Ramalingaswami. V.: Arch. Pathol. 88, 631 (1969)
- 264 Brooks. S. E. H.: Miller. C. G.; McKenzie, K.: Audretsch. J. J.; Bras. G.: Arch. Pathol. 89, 507 (1970)
- 265 APHasany. M.: Mohamed. A. A.: Arch. Dis. Child. 45, 722 (1970)
- 266 MeLe~in~ E. K.: Pharmacol. Rev. 22. 429 (1970)
- 267 McLean, E. K.: Isr. J. Med. Sci. 10, 436 (1974)
- 268 Moliabbat. 0.: Srivastava. R. N.; Younos. M. S.: Sediq, G. G.: Merzad. A. A.: Aram. G. N.: Lancet 2. 269 (1976)
- 269 Lyford. C. L.: Vergara, G. G.: Moeller, D. D.: Gastroenterology 70. 105 (1976)
- 270 Stillinan. A. E.; Huxtable, R. J.; Consroe. P.: Kohnen. P.; Smith. S.: Gastroenterology 73, 349 (1977)
- 271 Aikat. B. K.: Bhusnurmath, S. R.; Datta, D. V.; Chuttani. P. N.: Ind. J. Pathol. Microbiol. 21, 203 (1978)
- 272 Datta. D. V.: Kituroo, M. S.: Mattocks. A. R.~ Aikat. B. N.~ Chhuttani, P. N.: Postgrad. Med. J. 54, 511 (1978)
- 273 Srivastava. R. N.; Mohabbat. 0.; Ghani. A. R.: Aram. G. N.: Ind. Paediatr. 15, 143 (1978)
- 274 Arora. R, R.: Pyarelal; Ghosh, T. K.; Mathur, K. K.; Tandon, B. N.: J. Comm. Dis. (India) 13, 147 (1981)
- 275 Gharem. L: Hersliko, C.: Isr. J. Med. Sci. 17, 339 j1981)
- 276 Tanner. M. S.: Portmann, B.: Arch. Dis. Child. 56, 4 (1981)
- 277 Kumana. C. R.: Ng, M.; Li% H. L; Ko, W,; Wu, P. C.; Todd, D.: Lancet 2, 1360 (1983)
- 278 Kumana. C. R.: Ng. M.; Lin, H. L; Ko, W.; Wu. P. C.; Todd. D.: Gut 26, 101 (1985)
- 279 Fox. D. W.: Montgomery, C. H.: Bergeson, P. S.; Jarrett. P. B.; Stillman, A. E.: Huxtable. R. J.: J. Pediatr. 93, 980 (1978)
- 280 Schoental. R.: Br. Med. J. 2, 351 (1955)
- 281 Schoental. R.: Lancet 2, 176 (1974)
- 282 Heath. D.: Shaba. J.: Williams. A.: Smith, P.: Kornbe. P.: Thorax 30. 399 (1975)
- 283 Levine, 0. R.; Harris, R. C.; Blance, W. A.; Mellins, R. B.: J. Pediatr. 83, 964(1973)
- 284 Fishman, A. P.: Circulation Res. 35, 657 (1974)
- 285 Lafranconi, W. M.; Huxtable, R. L: Drug Metab. Drug Interact. 3, 271 (1981)
- 286 Lafranconi, W. M.; Duhamel, R. C.; Brendel, K.; Huxtable, R. L: Biochem. Pharmac91. 33. 191 (1984)
- 287 Koch. H. P.: Pharmazie 47, 531 (1992)
- 288 Koch, H. P.~ Plobner, K.; Trojan, M.: Pharmazie 49, 934 (1994)
- 289 Pavlica, D.; Samuel, L: Br. J. Cancer 24, 22 (1970)
- 290 Williarns, A. 0.; Edington, G. M.; Obakponovwe, P. C.: Br. J. Cancer 21, 474 (1967)
- 291 Schoental, R.: Cancer. Res. 28, 2237 (1968)
- 292 Schoental, R.: Coady, A.: East Afr. Med. J. 45, 577 (1968)
- 293 Cook, J. W.; Duffy, E.; Schoental, R.: Br. J. Cancer 4, 405 (1950)
- 294 Schoental, R.: Head, M. A.; Peacock, P. R.: Br. J. Cancer 8, 458 (1954)
- 295 Schoental. R.: Nature 227, 401 (1970)
- 296 Harris, P. N.; Chen, K. K.: Cancer Res. 30, 2881 (1970)
- 297 Schoental, R.: Fowler, M. E.; Coady, A.: Cancer Res. 30, 2127 (1970)
- 298 Schoental. R. Cavanagh, J. B.: J. Nad. Cancer Inst. 49, 665 (1972)
- 299 Svoboda, D. L; Reddy, J. K.: Cancer Res. 32, 908 (1972)
- 300 Hirono, L; Shimizu, M.; Fushimi, K.; Mori, H.; Kato, K.: Gann Monogr. Cancer Res. 64. 527 (1973)
- 301 Svoboda. D.: Reddy, J. K.: J. Nad. Cancer Inst. 53, 1415 (1974)
- 302 Allen. J. R.; Hsu, I. C.; Carstens, L. A.: Cancer Res. 35, 997 (1975)
- 303 Hirono, L: Mori. H.~ Yamada, K.; Hirata, Y.; Haga, M.; Tatematsu, H.; Kanie. S.: J. Nati. Cancer Inst. 58, 1155 (1977)
- 304 Anon.: National Cancer Institute (Bethesda, MI) U S NTIS, PB. Rep. Gov. Rep. Ann. Ind. 78, 216 (1978): C. A. 89, 124293e (1978)
- 305 Johnson, W. D. ~ Robertson, K. A.; Pounds, J. G.; Allen, J. R.: J. Natl. Cancer Inst. 61. 85 (1978)
- 306 Rao, M. S.: Reddy, J. K.: Br. J. Cancer 37, 289 (1978)
- 307 Kultara, K.~ Takanashi, H.; Hirono, L; Furuya, T.; Asada, Y.: Cancer Lett. 10. 117 (1980)
- 308 Mattocks, A. R.; Cabral, J. R. P.: Cancer Lett. 17, 61 (1982)
- 309 Peterson. J. E.: Jago, M. V.; Reddy, J. K.; Jarrett. R. G.: J. Nati. Cancer Inst. 70. 381 (1983)
- 310 Hirono, L: Ueno. L: Aiso, S.; Yamaji, T.; Haga, M.: Cancer Lett. 20, 191 (1983)
- 311 Culvenor, C. C. J.: J. Toxicol. Environ. Health 11, 625 (1983)
- 312 White. R. D.; Krumperman, P. H.; Cheeke, P. R.; Buffier. D. R.: Toxicol. Lett. 15. 25 (1983)
- 313 White. R. D.; Krumperman, P. H.; Cheeke, P. R.; Deinzer. M. L.; Buffier, D. R.: J. Animal. Sci. 58, 1245 (1984)
- 314 Rubiolo. P.: Picters. L.; Calomme, M.; Bicchi, C.; V1ietirick, A.; Van den Berghe, D.: Mutat. Res. 281, 143 (1992)
- 315 Clark. A. M.: Z. VererbungsI. 91, 74 (1960)
- 316 Avanzi. S.: Carvologia 14, 251 (1961)
- 317 Cook. L. M.: Holt. A. C. E.: J. Genet. 59, 273 (1966)
- 318 Bick. Y. A. E.: Culvenor, C. C. J.; Jago, M. V. E.: Cytobios. 14, 151 (1975)
- 319 De Paula Ramos, A. L.; Marques, E. K.: Rev. Brasil. Genet. 4, 279 (1978)
- 320 Weltner, F. C.~ Thiel. P. G.~ van Rensburg, S. J.: Mutat. Res. 66,187 (1979)
- 321 Yamanaka. H.~ Nagao, M.; Sugimura, T.; Furuya, T.; Shirai. A.; Matsushima, T.: Mutat. Res. 68,211 (1979)
- 322 Takanashi. H.~ Unteda, M.; Hirono, L: Mutat. Res. 78,67 (1980)
- 323 Williams, G. M.; Mori, H.; Hirono, L; Nagao. M.: Mutat. Res. 79,1 (1980)
- 324 Green, C. E. ~ Segall. H. L; Byard, J. L.: Toxicol. Appl. Pharmacol. 60, 176 (1981)
- 325 Robertson. K. A.: Cancer Res. 42, 8 (1982)
- 326 Candrian. U.: Luethy, L; Graf, U.; Schlatter, C.: 17d. Chem. Tox. 22, 223 (1984)
- 327 Petry, T. W.: Bowden, G. T.; Huxtable, R. J.; Sipes, 1. G.: Cancer Res. 44, 1505 (1984)
- 328 Mori. H.: Sugie. S.: Yoshimi, N.; Asada, Y.; Furuya, T.; Williams, G. M.: Cancer Res. 45. 3125 (1985)
- 329 Candrian, U.~ Luethy, L; Schlatter, C.: Chern. Biol. Interact. 54, 57 (1985)
- 330 Kraus. C.~ Abel. G.~ Schimmer. 0.: Planta Med. 89 (1985)
- 331 Bruggeman, 1. M.: van der Hoeven, J. C. M.: Mutat. Res. 142, 209 (1985)
- 332 Griffin. D. S.: Segall, H. J. D.: Toxicol. Appi. Pharmacol. 86, 227 (1986)
- 333 Da Rocha. M. S. C.; De Azevedo, J. L.: Rev. Brasil. Genet. 9. 393 (1987)
- 334 Gimmier-Luz, M. C.; Marques, E. K.; Johnston, R. C.: Rev. Brasil. Genet. 10, 31 (1987)
- 335 Zijlstra. J. A.: Vogel, E. W.: Mutat. Res. 202, 251 (1988)
- 336 Reed. R. L.: Ahern. K. G.; Pearson, G. D.; Bultler, D. R.: Carcinogenesis 9, 1355 (1988)
- 337 Gimmler-Luz. M. C.; Erdtmann, B.; Balbueno, R. A.: Mutat. Res. 241, 297 (1990)
- 338 Hiricks, J. R.~ Kim, 11. Y.; Segall, H. J.; Molyneux, R. L; Sternitz, F. R.; Coulombe jr.. R. A.: Toxicol. Appl. Pharmacol. 111, 90 (1991)
- 339 Mueller, L.: Kasper, P.; Kaufmann, G.: Mutat. Res. 282, 169 (1992)
- 340 Frei, H. J.; Luethy, J.; Brauchli, L; Zweifel, U.; Wuergler, F. E.; Schlatter, C.: Chem. Biol. Interact. 83, 1 (1992)
- 341 Green, C. R.; Christie, G. S.: Brit. J. Exp. Pathol. 42, 369 (1961)
- 342 Peterson, J. Il.: Jago, M. V.: J. Pathol. 131, 339 (1980)
- 343 Brink, N. G.: Mutat. Res. 104, 105 (1982)
- 344 Schoental, R.; Mattocks, A. R.: Nature 185, 842 (1960)
- 345 Culvenor, C. C. J.; Edgar, J. A.; Jago, M. V.; Outteridge, A.; Peterson, J. E.; Smith, L. W.: Chern. Biol. Interact. 12, 299 (1976)
- 346 Mattocks, A. R.: Tox. Lett. 14, 111 (1982)
- 347 Mattocks, A. R.: Nature (London) 217, 723 (1968)
- 348 Mattocks, A. R.: J. Chem. Soc. 1155 (1969)
- 349 Culvenor, C. C. J.; Downing, D. T.; Edgar, J. A.; Jago, M. V.: Ann. N. Y. Acad. Sci. 163, 837 (1969)
- 350 Jago, M. V.; Edgar, J. A.; Smith, L. W.; Culvenor, C. C. L: Mol. Pharmacol. 6, 402 (1970)
- 351 Mattocks, A. R.; White, 1. N. H.: Chem. Biol. Interact. 3, 383 (1971)
- 352 Mattocks, A. R.: Xenobiotica 1, 563 (1971)
- 353 Mattocks, A. R.: Chem. Biol. Interact. 5, 227 (1972)
- 354 Mattocks, A. R.; Bird, L: Tox. lett. 16, 1 (1983)
- 355 Schoental, R.: Nature (London) 179, 361 (1957)
- 356 Culvenor, C. C. J.; Dann, A. T.; Dick, A. T.: Nature (London) 195, 570 (1962)
- 357 Mattocks, A. R.: Nature (London) 217, 723 (1968)
- 358 Culvenor, C. C. J.; Downing, D. T.. Edgar, J. A.; Jago. M. V.: Ann. N. Y. Acad. Sci. 163, 837 (1969)
- 359 Black, D. N.; Jago, M. V.: Biochem. J. 118, 347 (1970)
- 360 Culvenor, C. C. J.; Edgar, J. A.; Smith, L. W.; Tweeddale, H. J.: Aust. J. Chem. 23, 1853 (1970)
- 361 Mattocks, A. R.: Chem. Biol. Interact. 35, 31 (198 1)
- 362 Mattocks, A. R.; Bird, L: Chem. Biol. Interact. 43, 209 (1983)
- 363 Culvenor, C. C. J.; Edgar, J. A.; Smith, L. W.: Jago. M. V.; Peterson, J. E.: Nature (London) 232, 255 (1971)
- 364 Culvenor, C. C. L; Edgar, J. A.; Smith, L. W_ Peterson, J. E: Nature New Biol. 229, 255 (1971)
- 365 Culvenor, C. C. J.; Jago, M. V.: in Grover, P. L. (ed.) Carcinogenic Plant Products and DNA. Vol. 1, p. 161, CRC Press, Boca Raton, Florida 1979
- 366 Mattocks, A. R.; Jukes, R.: Nat. Tox. 1, 89 (1992)
- 367 v. Petersenn, A.; Roeder, E: Meeting Germ. French. Pharmaccut. Soc.. Strasbourg, 19-22. 09.1991, Arch. Pharm. 324, 744 p88 (1991)
- 368 Kedzierski, B.; Buhler, D. R.: Toxicol. Lett. 25, 115 (1985)
- 369 Kedzierski, B.; Buffier, D. R.: Chem. Biol. Interact. 57. 217 (1986)
- 370 Robertson, K. A.; Seymour, J. L.; Hsia, M. T,; Alien, J. R.: Cancer Res. 37, 3141 (1977)
- 371 Estep, J. E_ Lam~, M. W.; Jones, A. D.; Segall, H. J.: Toxicol. Lett. 54, 61 (1990)
- 372 Huxtable, R. L; Bowers, R.; Mattocks, A. R.; Michnicka, M.: Biol. React. Intermed. 4, 605 (1990)
- 373 Mattocks, A. R.; Jukes, R.: Chem. Biol. Interact. 75. 225 (1990)
- 374 Mattocks, A. R.; Jukes, R.: Toxicol. Lett. 63, 47 (1992)
- 375 Black, D. N.; Jago, M. V.: Biochem. J. 118, 347 (1970)
- 376 White, I. N. H.; Mattocks, A. R.: Biochem. J. 128. 291 (1972)
- 377 Schoental, R.: FEBS Lett. 61, 111 (1976)
- 378 Curtain, C. C.; Edgar, J. A.: Chem. Biol. Interact. 13. 243 (1976)
- 379 Robertson, K. A.: Cancer Res. 42, 8 (1982)
- 380 Mattocks, A. R.: Bird, L: Toxicol. Lett. 16, 1 (1983)
- 381 Wickramanayake, P. P.: Arbogast, B. L.; Buffier, D. R.: Deinzer, M. L.; Burlingame, A. L.: J. Am. Chem. Soc. 107, 2485 (1985)
- 382 Candrian, U.; Luethy, J.; Schlatter, C.: Chem. Biol. Interact. 54, 57 (1985)
- 383 Karchesy, J. J.; Arbogast, B.; Deinzer, M. L.: J. Org. Chem. 52,3867 (1987)
- 384 Reed, R. L.; Ahern, K. G.; Pearson, G. D.; Buhler, D. R.: Carcinogenesis 9, 1355 (1988)
- 385 Weidner, M. E; Millard, J. T.; Hopkins, P. B.: J. Am. Chem. Soc. 111, 9270 (1989)
- 386 Mattocks, A. R.; Jukes, R.: Chem. Biol. Interact. 76, 19 (1990)
- 387 Niwa, H.; Ogawa, T.; Okamoto, 0.: Yamada, K.: Tetrahedron Lett. 32, 927 (1991)
- 388 Segall, H. J.;.Wilson, D. W.; Dallas, J. L.; Haddon, W. E: Science 229, 472 (1985)
- 389 Mattocks, A. R.; White, 1. N. H.: Nature, New Biol. 231, 114 (1971)
- 390 Styles, J.; Ashby, L; Mattocks. A. R.: Carcinogenesis 1, 161 (1980)
- 391 Kupchan, S. M.; Doskotch, R. W.; Vanevenshoven, P. W. J.: J. Pharm. Sci. 53, 343 (1964)
- 392 Kupchan, S. M.; Suffness, M. T. L: J. Pharm. Sci. 56~ 541 (1967)
- 393 Culvenor, C. C. J.; J. Pharm. Sci. 57, 1112 (1968)
- 394 Kovach, J. S.; Arnes, M. W Powis, G.; Moertel, C. G.: Halm, R. G.; Creagan, E. T.: Cancer Res. 39, 4540 (1979)
- 395 Powis, G.; Ames, M. M.. Kovach, J. S.: Cancer Res. 39, 3564 (1979)
- 396 Miser, J. S.; Miser, A. W.; Smithson, W. A.; Coccia, P. E: Ames. M. M.; Davis, D. M.; Hughes, C. S.: Gilchrist, G. S.: Proc. Am. Soc. Clin. Oncol. 1, 137 (1982)
- 397 Kovach, J. S.; Moertel, C. G.; Halm, R. G.: Proceed. Am. Ass. Cancer Res. 20, 357 (1979)
- 398 Letendre, L.; Smithson, W. A.; Gilchrist, G. S.; Burgert, E. 0.~ Hoagland, C. H.; Ames, M. M_ Powis, G.; Kovach, J. S.: Cancer 47,437 (1981)
- 399 Nicols, W. C.: Moertel, C. G.; Rubin, L; Schutt. A. L; Britell, J. C.: Cancer Treatm. Rep. 65, 337 (1981)
- 400 Ohnuma, T.; Sridhar, K. S.; Ratner, L. H.; Holland, J. E: Cancer Treatm. Rep. 66, 1509 (1982)
- 401 Letendre, L.: Ludwig, J.; Perrault, L; Smithson. W. A.: Kovach, J. S.: Cancer 54, 1256 (1984)
- 402 Cook, B. A.; Sinnhuber, J. R.: Thomas, P. J.; Olson, T. A.; Silverman, T. A.; Jones, R.; Whitehead, V. M.; Ruymann, F. B.: Cancer 52. 61 (1983)
- 403 Winton, E. F.; McCue, P. A.: Cancer Treatm. Rep. 70, 933 (1986)
- 404 Suffness, M.: Cordell, G. H.: Antitumour Alkaloids, in: Brossi. A. (ed.): The Alkaloids, Vol. 25, Academic Press, New York 1985
Received July 14, 1994
Accepted July 17, 1994
Prof. Dr. E. Roeder
Pharmazeutisches Institut der Universität Bonn
D-53121 Bonn-Endenich
Medicinal plants in Europe containing pyrrolizidine alkaloids was written by Prof. Dr. E. Röder and published in the journal "Pharmazie" 50 (1995), pages 83-98.