 Parts used - Botanical analysis - Common names - Specific description - Allied species - Characteristics - Constituents - Anemonin - Anemonic acid - Anemoninic acid - Anemonol (Oil of Anemone) - Oil No. 2- Pharmacopoeial history - Preparations - Medical history - Medical uses - Homoeopathic uses - Pharmaceutical and Medical References
Parts used - Botanical analysis - Common names - Specific description - Allied species - Characteristics - Constituents - Anemonin - Anemonic acid - Anemoninic acid - Anemonol (Oil of Anemone) - Oil No. 2- Pharmacopoeial history - Preparations - Medical history - Medical uses - Homoeopathic uses - Pharmaceutical and Medical References
PARTS USED.—The fresh bulbous base and flowering tops of Ranunculus bulbosus Linn.
Natural Order Ranunculaceae, Tribe Ranunculeae.
BOTANICAL ANALYSIS.—Roots, fleshy, fibrous. Stem erect from the bulbous base, branched, hairy. Leaves mostly radical, few cauline, petiolate, three-divided; divisions, lateral nearly sessile, terminal stalked, all more or less three-parted and incisely toothed and lobed; petioles, sulcate, grooved on the upper side, amplexical, the bases of those of the radical leaves fleshy and united, forming a bulbous base to the plant. Flowers terminal, slender pedunculate. Sepals, five, reflexed, hairy externally. Petals, five, orbicular, veiny, spreading, having a small nectariferous cavity on inner side at the base, covered with a small, wedge-shape, emarginate scale. Stamens numerous. Pistils numerous, in a head. Fruit, a globular head of achenes, tipped with short beaks.
COMMON NAMES.—The proper common name for all species of Ranunculus is Crowfoot, [In this country the name Crowfoot is misapplied often to Geranium maculatum, which is known to many root diggers and dealers under this name.] from the shape of the leaves of some species which resembles that of a crow's foot. Ranunculus bulbosus should be properly designated as Bulbous Crowfoot.
This species, and others that have large yellow flowers, are popularly known as Buttercups [This name is not derived from butter and cup, but is a corruption of the old English Button-cop, meaning bachelor's buttons, which was given to the double, cultivated variety of the plant.—PRIOR.] in this country. In England they are called also King-cups, Gold-cups, Gilt-cups, Gold-knobs.
They are occasionally called Yellow Weed and Meadow Bloom, from the yellow flowers; Blister Weed, from their acrid properties; and Burrwort because of the burr-like fruit, which, however, is not enough of a burr to justify the name.
Ranunculus bulbosus is sometimes called, in England, Saint Anthony's Turnip, or Saint Anthony's Rape, from the acrid bulbous base.
 SPECIFIC DESCRIPTION.—Ranunculus bulbosus is an erect herbaceous plant, growing about a foot high. It is a native of Europe, but has been naturalized, and is very common in fields and in sandy soil in the Atlantic States, though rare in the interior of the country. In some places in the East it is a great pest to the farmers, and so common that when in bloom the fields present a mass of yellow. The characteristic of the plant is the bulbous base, which is well shown in our engraving (Plate VII.). This differs from the true bulbs of plants; it is really the bases of the leaves and stems, grown together and enlarged by the accumulation of nutritious juices. It is the storehouse of the plant, in which is stored each summer the nutriment that the plant uses to grow and produce flowers next spring.
SPECIFIC DESCRIPTION.—Ranunculus bulbosus is an erect herbaceous plant, growing about a foot high. It is a native of Europe, but has been naturalized, and is very common in fields and in sandy soil in the Atlantic States, though rare in the interior of the country. In some places in the East it is a great pest to the farmers, and so common that when in bloom the fields present a mass of yellow. The characteristic of the plant is the bulbous base, which is well shown in our engraving (Plate VII.). This differs from the true bulbs of plants; it is really the bases of the leaves and stems, grown together and enlarged by the accumulation of nutritious juices. It is the storehouse of the plant, in which is stored each summer the nutriment that the plant uses to grow and produce flowers next spring.
 The leaves are mostly radical, and are borne on succulent, grooved stalks. The flowers appear in May, and are about an inch in diameter, and of a deep glossy yellow. They terminate the stems and branches.
The leaves are mostly radical, and are borne on succulent, grooved stalks. The flowers appear in May, and are about an inch in diameter, and of a deep glossy yellow. They terminate the stems and branches.
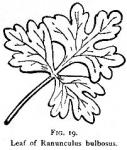
This species can readily be distinguished from the related species of Ranunculus by its bulbous base, by the stalked terminal division of the leaf (see Fig. 19), and by the early flowers.
ALLIED SPECIES.—The genus Ranunculus comprises about 150 species found in all countries, but most abundant in the temperate regions of the Northern Hemisphere. All possess more or less of the acrid properties.
Nearly 75 species and varieties are found in this country. In addition to Ranunculus bulbosus, only the following, however, deserve special mention.
 Ranunculus Acris Linn.—Like Ranunculus bulbosus, this is also a foreigner which has established itself much too firmly in the Eastern States. The two species somewhat resemble each other in general appearance. The Ranunculus acris [The plant is illustrated in Woodville's Medical Botany, Vol. III., p. 482, Plate 172, and in Stephenson and Churchill's Medical Botany, Vol. II., Plate 82.] can be readily distinguished by the following characters: The absence of the bulbous base; it is a taller plant, about two feet high; the flowers are on more slender stalks, and are of a lighter yellow color, and appear two months later, in July and August; the leaves are nearly orbicular, the divisions all sessile.
Ranunculus Acris Linn.—Like Ranunculus bulbosus, this is also a foreigner which has established itself much too firmly in the Eastern States. The two species somewhat resemble each other in general appearance. The Ranunculus acris [The plant is illustrated in Woodville's Medical Botany, Vol. III., p. 482, Plate 172, and in Stephenson and Churchill's Medical Botany, Vol. II., Plate 82.] can be readily distinguished by the following characters: The absence of the bulbous base; it is a taller plant, about two feet high; the flowers are on more slender stalks, and are of a lighter yellow color, and appear two months later, in July and August; the leaves are nearly orbicular, the divisions all sessile.
 Ranunculus Repens Linn.—This plant is also related to the two species previously described. It is a native of this country, though found in Europe. It grows in woodland pastures and along streams in most sections of the country. The stems are decumbent at the base. The flowers appear in the spring, and are of a glossy yellow color. After flowering, the plant sends out from its base creeping runners, whence the specific name. The leaves are three-parted, and the leaflets all stalked. Ranunculus repens possesses the acrid principle only in a mild degree. It is called Creeping Crowfoot.
Ranunculus Repens Linn.—This plant is also related to the two species previously described. It is a native of this country, though found in Europe. It grows in woodland pastures and along streams in most sections of the country. The stems are decumbent at the base. The flowers appear in the spring, and are of a glossy yellow color. After flowering, the plant sends out from its base creeping runners, whence the specific name. The leaves are three-parted, and the leaflets all stalked. Ranunculus repens possesses the acrid principle only in a mild degree. It is called Creeping Crowfoot.
 Ranunculus sceleratus Linn.—This plant differs much in appearance from those previously described, and belongs to a separate section of the genus. The flowers are small and inconspicuous, and the fruit-heads are cylindrical. The stem is erect, smooth, succulent and hollow. The leaves are all three-parted, or three-lobed, with few-toothed lobes. This plant grows in wet situations, and is found also in Europe. It is probably the most acrid of our native species, and is called Cursed Crowfoot.
Ranunculus sceleratus Linn.—This plant differs much in appearance from those previously described, and belongs to a separate section of the genus. The flowers are small and inconspicuous, and the fruit-heads are cylindrical. The stem is erect, smooth, succulent and hollow. The leaves are all three-parted, or three-lobed, with few-toothed lobes. This plant grows in wet situations, and is found also in Europe. It is probably the most acrid of our native species, and is called Cursed Crowfoot.
Ranunculus abortivus Linn.—This plant is a common weed in fields and around dwellings in most parts of the country. It bears inconspicuous yellow flowers in spring, which are followed by small globular fruit-heads, and it withers away and dies in June, shortly after the fruit has ripened. There is a marked contrast in the shape of the leaves: the radical are round-cordate, crenate, and petiolate; the upper are sessile, three-parted, with linear, entire divisions; the intermediate leaves partake more or less of the character of both forms.
As this plant is common in most sections, and possesses in a marked degree the acrid principle [This is the species from which we derived the acrid principle for our experiments.] of the family, we introduce a cut by which it can be at once recognized. (See Fig. 23, next page.)
 CHARACTERISTICS.—The entire plant of most species of Ranunculus is acrid, the full-grown, green fruit and the root being especially active. To the taste they are peppery and pungent, reminding us of mustard or horse-radish. If the bruised plant be bound upon the skin, like mustard, it irritates, inflames and blisters. The crushed plant emits a vapor which irritates and inflames the eyes. Boiling with water dissipates the acrid principle, and some species of Ranunculus are eaten as greens. The pulp of those most acrid, after being boiled by us, proved to be free from acridity.
CHARACTERISTICS.—The entire plant of most species of Ranunculus is acrid, the full-grown, green fruit and the root being especially active. To the taste they are peppery and pungent, reminding us of mustard or horse-radish. If the bruised plant be bound upon the skin, like mustard, it irritates, inflames and blisters. The crushed plant emits a vapor which irritates and inflames the eyes. Boiling with water dissipates the acrid principle, and some species of Ranunculus are eaten as greens. The pulp of those most acrid, after being boiled by us, proved to be free from acridity.
CONSTITUENTS.—When the plant is bruised (preferably the green fruit), and treated with sulphuric ether, the acrid volatile principle is abstracted, and may be obtained in an impure form by spontaneously evaporating the ether. This acrid principle is a volatile oil, and may be obtained easier and in a purer condition by distillation (see Oil of Anemone).
Anemonin is another substance accepted generally as existing in the acrid species of Ranunculus. If the pulp of the plant, after extraction with ether (or before), be exhausted with chloroform and this chloroformic solution evaporated spontaneously, an oily residue remains, free from crystals of anemonin, and which refuses to react with Fehling's test solution for glucose. This leads us to believe that anemonin is a product of the action of boiling water on the plant rather than an educt.
If the bruised root of the plant be mixed with water, it forms a milky emulsion-like liquid, and, upon heating, it coagulates at less than the boiling point.
When the plant is covered with water and distilled, pungent acrid vapors escape. Upon condensation a transparent liquid of a pungent color and a peppery taste is obtained. After standing a short time, the liquid deposits white flocculent matter. This distilled water is the interesting product of the numerous plants yielding anemonin. [The following plants may be named as having been used in making anemonin; Anemone nemorosa, Anemone pratensis, Anemone Pulsatilla, Anemone patens Linn. var. Nuttalliana Gray, Ranunculus bulbosus, Ranunculus Flammula, Ranunculus sceleratus. We have found it in Ranunculus recurvatus, and the plant used in this line of experiments, Ranunculus abortivus. Doubtless all the plants of the Ranunculaceae containing a volatile acrid substance are of a like nature.]
Examination of the Distillate.—Into a twenty-five gallon copper still, [Experiments on a small scale were unsatisfactory. The volatile oils were obtained in minute proportion, but the anemonin and anemonic acid were scarcely to be found. We made several batches on a large scale, with good results.] thirty pounds of Ranunculus abortivus was placed, and covered with water. This was distilled, with the following result:
- First gallon, colorless, clear, neutral, nearly tasteless.
- Second gallon, colorless, neutral, pungent, acrid, with flocculent concrete substance throughout it.
- Third, fourth, fifth and sixth gallon, corresponding to second in properties.
The odor of each fraction was herby as well as pungent. The last portion had a burnt odor. The amount of concrete flocculent matter that formed in the distillate rather increased as the process continued.
Chloroform [Previous experiments had shown us that chloroform is the solvent for the principles contained in the distilled water. Sulphuric ether has been used by others, but, owing to the fact that water dissolves ether and thus forms a solvent for the volatile oil, those who use ether to separate the oil, labor under a disadvantage. Again, ether is an inferior solvent for anemonin, and hence is a poor agent to obtain that substance. These objections are overcome by chloroform, which extracts all of the volatile oils from the water as well as the anemonin and anemonic acid.] was then well shaken with the water, separated, and filtered. The water was now clear, and nearly tasteless. The chloroform was of a light straw color in bulk, transparent in small amounts. Small portions, by evaporation, left an acrid oil mixed with crystals. This chloroformic liquid was then cautiously distilled until it was concentrated to a small bulk. The residue was of a yellow color. The re-condensed chloroform did not contain the acrid volatile oil of anemone.
Upon permitting the chloroformic liquid within the retort to remain excluded from the air, [Mr. O. L. Erdmann (see Am. Jour. of Pharm., 1859, p. 441) refers to the formation of this precipitate (anemonic acid), where a solution of anemonin is exposed to the air. He questions as to whether it is a product of oxidation, and will take place only in contact with water or air. Our experiments demonstrate that it is not necessary to admit the atmosphere in order that anemonin should disintegrate.] it deposited in twelve hours a flocculent substance of a drab color (impure anemonic acid), the liquid becoming colorless, but retaining its acridity unimpaired.
This liquid, by spontaneous evaporation, deposited a mass of crystals (anemonin), some amorphous material (anemonic acid), an acrid volatile oil which evaporated next after the chloroform, and a peculiar fragrant volatile oil which remained for some time after the acrid oil had vaporized.
If the chloroformic liquid be filtered, in a short time the deposit of anemonic acid again occurs, and will continue until most of the anemonin has perished.
Summary.—All parts of fresh Ranunculus yield, when bruised, a volatile principle that, from its resemblance to volatile oil of horse radish or mustard, may likely be identical with one of those substances.
Ether or chloroform will extract this oil from the fresh fruit, leaving an odorless pulp, which fails to produce the oil again upon moistening and exposure. Chloroform failed in our hands to extract anemonin or anemonic acid from any portion of the fresh plant, thus leading to the inference that these substances do not exist in the plant. [In endeavoring to extract proximate principles from fresh plants by means of chloroform, the operator must bear in mind the fact that chloroform will scarcely come in contact with these principles while the plant is saturated with water. If the substance to be obtained is insoluble in ether, wash the magma first with ether, then use the chloroform.]
Water distilled freshly from the plant is acrid, transparent, and free from flocculi. It does not react with Fehling's solution. In a few days it deposits a white substance which chloroform extracts from it. This substance is crystalline. The crystals react with Fehling's solution with an abundant deposit of cuprous oxide. This is anemonin. The indications then, are:
1st. That plants yielding oil of anemone produce it by the decomposition of proximate principles, after the manner of the production of oil of bitter almonds and volatile oil of mustard. [It must be stated, however, that after drying the pulp and triturating it with emulsion of sweet almonds, the oil failed to be reproduced. With white mustard seed the experiment seemed to be more satisfactory, but owing to the fact that moistened pulp of white mustard emits a pungent vapor, we could not decide positively. The indications are that the ferment of Ranunculus is not identical with either of those we have named.]
2d. Anemonin and anemonic acid are products, and not educts.
ANEMONIN (C15H12O6).—This substance has received attention from Heyer, Vauquelin and Robert, Schwarz, Rabenhorst, Löwig and Weidmann, Fehling, Müller, Erdmann, and perhaps others.
In 1771, Störk distilled a mixture of Anemone Pulsatilla and water. He noticed a flocculent substance in the condensed liquid, and called it Pulsatilla Camphor; and this substance was again noticed (1779) by Heyer. In our opinion, this is the earliest record of anemonin, for, together with the so-called anemonic acid, it partially separates from such a distillate.
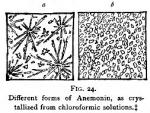
Owing to the discordant reports from those who have experimented with anemonin, we must conclude that either different substances, or anemonin in an impure condition, was sometimes employed; and we are inclined to favor the latter opinion. Some persons may argue that, inasmuch as varying forms of crystals have been reported from Anemone nemorosa and Anemone Pulsatilla, it is probable that anemonin is not identical as obtained from different species of plants. This view we do not hold to be necessarily correct, since, according to our experiments, anemonin from Ranunculus abortivus may crystallize either in plates, in delicate acicular needles, or in clumps of grainy masses, varying in appearance and perfection of crystals, in accordance with the solvent and the impurities (see Fig. 24), although perhaps of the same fundamental form.
 [These were both made from one solution, the difference resulting from depth of liquid and rapidity of evaporation.]
[These were both made from one solution, the difference resulting from depth of liquid and rapidity of evaporation.]
In our paper on American pulsatilla (p. 30), and Anemone nemorosa (p. 22), we referred to anemonin as the important constituent of those plants, promising, however, to consider it later. [Owing to the early time of year, we could not then obtain the plants to prepare it.] We will first give an arrangement of the properties of anemonin, as we find them recorded. [The experiments that have been made with anemonin were mostly instituted prior to the year 1864. Therefore Gmelin's Chemistry, Vol. XVI., may be consulted with advantage by those who are interested, for since that time there have been few additions.]
- Erdmann.—Anemonin results when by age the volatile oil of the Anemones decomposes. It is soluble in chloroform.
- Vauquelin.—Anemonin volatilizes undecomposed when heated in a glass tube, condensing to an oil which concretes, a small amount of a brown resinous substance remaining. Anemonin is slightly soluble in cold water, more so in boiling water, crystallizing from the hot solution when it cools.
- Heyer.—Anemonin yields, when heated, a colorless watery distillate of a peppery taste; a yellow empyreumatic sublimate, soluble in alcohol; and a charcoal residue. Anemonin burns in a flame without residue. It dissolves in hot oil of lavender and hot palm oil.
- Heyer and Schwarz.—Anemonin is deposited with anemonic acid from the water which distills from Anemone Pulsatilla (Heyer) and Anemone nemorosa (Schwarz).
- Heyer and Robert.—Anemonin is heavier than water, friable, inodorous; has a fatty taste when crystallized, but if melted is biting and burning, benumbing the tongue.
- Grailich and Lang.—Colorless, mostly tabular shining prisms belonging to the right prismatic system; short rhombic prisms (Anemone pratensis); long prisms (Anemone nemorosa).
- Löwig and Weidmann.—Anemonin dissolves without decomposition in sulphuric acid. Hydrochloric acid converts it into anemoninic acid. It dissolves in aqueous alkalies. Baryta water converts it, two molecules of water being taken up, into anemoninic acid (Fehling contra), which forms brown amorphous salts, of which the lead, mercury and silver salts are insoluble in water.
- Fehling.—Anemonin is neutral. Softens at 15° C, giving off water and a pungent vapor, then turns yellow and decomposes above 300° C. Heated with nitric acid, it forms oxalic acid. Heated with dioxide of manganese and sulphuric acid, it forms formic acid. Solutions of the alkalies and baryta water dissolve it with a yellow color, and they are neutralized. Oxides of lead and silver form compounds with anemonin that crystallize on cooling, and which are sparingly soluble in cold alcohol, readily in boiling alcohol, crystallizing from the hot solution upon cooling. Anemonin is insoluble in cold ether and slightly in boiling. Sulphuric acid carbonizes it. If quickly heated in chlorine gas, hydrochloric acid and a volatile oil result. Anemonin is an acrid poison.
In reviewing the foregoing, it will be found that by mutual agreement, anemonin is considered as a crystalline solid obtained by distillation from several species of the Ranunculaceae. It is also reported that an acrid volatile oil accompanies it; that, upon exposure to the atmosphere, anemonin decomposes, one of the products being a substance to which the name anemonic acid has been given. The only testimony concerning the theory of its production, is that of Erdmann, who states that the volatile oil decomposes by standing into anemonin and anemonic acid. In the main, our experiments agree with those of others; but the relationships of anemonin, anemonic acid and the volatile oil—all of which are obtained by distillation of the plant—have not been recorded as we view the subject. Erdmann approached the matter, but circumstances overlooked by him lead us to believe that anemonin and anemonic acid are not products of the decomposition of the volatile oil under the influence of water or oxygen. Therefore we will bring forward our experiments, and venture to formulate an opinion regarding this problem.
1st. When the distillate is obtained it is transparent, acrid and pungent. In a short time it becomes cloudy, and finally fills with clots of white amorphous matter. Upon agitation with chloroform, this matter dissolves. Upon evaporation of the chloroform, it crystallizes. This is anemonin. However, the production of the anemonin does not seem to decrease the acridity of the liquid. The volatile oil does not disappear if the container be secured. Hence we doubt if anemonin results from decomposition of the oil. [In exposing the chloroformic solution, after agitation of chloroform with distilled water of the plant, crystals of anemonin separate as the chloroform and oils evaporate. It would be quite natural to suppose that the oil decomposed to produce it, as Erdmann did.]
2d. If crude anemonin be exposed to the atmosphere, it decomposes, a substance remaining, which is insoluble in chloroform or other solvent of anemonin; and during this change there is a constant evolution of a pungent vapor and a volatile oil.
We are of the opinion that anemonin alone will not decompose to form oil by exposure. Our experiments indicate that some substance accompanies it, and induces the change. We have noticed that after prolonged exposure of a chloroformic solution, when nearly all of the odor of oil of anemone had disappeared, a small amount of anemonin remained. This refused to decompose, and we can only explain the fact by supposing that the ferment had been exhausted. Pure anemonin is permanent. It seems difficult to isolate each of these substances, as like solvents act upon them, but if crystals of anemonin be well washed with sulphuric ether, they are obtained pure, and are then permanent in dry air.
3d. If a clear, colorless chloroformic solution of anemonin, as obtained from the aqueous distillate, be permitted to stand a short time, it becomes cloudy, and in a day, or less time, the substance called anemonic acid commences to separate. Filtration of the mixture clarifies the liquid, but decomposition again takes place, and will continue until the anemonin has mostly perished.
If a chloroformic solution of anemonin, as obtained from the distillate of the plant, be exposed in an evaporating dish, the chloroform quickly evaporates; finally only a pungent liquid (oil of anemone) remains, which, however is interspersed with crystals of anemonin. Gradually the oil evaporates and the anemonin disappears; the mass becomes acid and sour to the taste, assumes a yellowish color, becomes gummy (anemonic acid), and largely insoluble in the solvents for anemonin; and during this period there is a constant evolution of pungent vapors and of oils of anemone.
4th. If a chloroformic solution obtained from the distilled water of a plant which yields anemonin, be evaporated to dryness in a retort, the temperature of which can not rise above 82° C, the result will be as follows: First the chloroform distills; then the pungent substance, oil of anemone, with traces of chloroform; then these substances, free from chloroform, but after the chloroform has evaporated, if the heat be continued the semi-crystalline mass within the retort decomposes, and finally a white, tough magma remains, which is insoluble in all solvents for anemonin. This is anemonic acid.
Summary.—Anemonin is the product of the action of boiling water on the fresh herb of various species of the Ranunculaceae. It is associated with the volatile oils of anemone in the aqueous distillate obtained from these plants. It gradually separates from this distilled water in an amorphous condition, and when taken up by appropriate solvents may be crystallized. Anemonin, as associated with the substances accompanying it, is prone to decompose, one of the products being an insoluble amorphous body known as anemonic acid. This decomposition occurs either in contact with air and water, or excluded from it. Pure anemonin is stable.
To Prepare Anemonin.—Take any convenient quantity of the chloroformic liquid obtained (p. 60) by agitating chloroform with the distillate from a plant yielding anemonin. Place it in an evaporating dish, and by spontaneous evaporation, aiding the operation by gently fanning the liquid, [Liquids of this nature should not be breathed upon to evaporate them. The moisture of the breath condenses in them, owing to the low temperature induced by the rapid evaporation. This water produces a milkiness, and after the chloroform, ether, benzol or carbon disulphide evaporates, the water remains, often to spoil a crop of crystals.] dissipate the chloroform. As soon as the odor of chloroform disappears, and crystals form, wash the crystals repeatedly with sulphuric ether, specific gravity 0.750. Then collect on a filter paper, and dry them by exposure to the atmosphere.
Description of Anemonin.—Anemonin is white, odorless, tasteless. It crystallizes in groups of acicular needles, or in tabular form. The crystals are transparent and shining, but if washed with ether become semi-opaque and white. (See Fig. 24, a and b, both of which were prepared from the same solution under different conditions.) They do not evaporate upon exposure.
Cold sulphuric acid does not discolor them, but dissolves them slowly, forming a colorless liquid. Heat changes this to yellow, then red, and finally brown, with evolution of pungent fumes. (This agrees with Löwig, Weidmann and Müller, but differs with Fehling, who states that sulphuric acid carbonizes anemonin.)
Hydrochloric acid (cold) does not affect anemonin, but boiling dissolves it. (Löwig and Weidmann state that hot hydrochloric acid forms anemoninic acid with anemonin.)
Nitric acid (cold) neither decomposes nor dissolves anemonin, but hot nitric acid dissolves it with decomposition and evolution of nitrous oxide, forming a colorless or straw-colored liquid. Ammonia changes this to yellow. (Fehling states that oxalic acid results as one of the products of this reaction.)
Ammonia water, cold or hot, refuses to affect it.
Heated in a retort, anemonin fuses with decomposition, turns yellow, then chars, a carbonaceous mass remaining. Pungent, irritating vapors are evolved during the operation, and upon condensation an oily liquid is obtained, which possesses the sensible properties of the acrid volatile oil of anemone, irritating the skin in like manner, and presenting the same odor. [We state (p. 64) that if a chloroformic solution of the volatile substances obtained from a plant yielding anemonin be distilled to dryness, the anemonin decomposes, crude anemonic acid resulting while oil of anemone distills. If this anemonic acid be heated under a direct flame, further decomposition follows, accompanied with the production of a substance like the afore named oil, which reminds us of acrolein, being not inferior to that substance in pungency and acridity.] It is neutral to litmus. Vauquelin and some others have stated, or accepted, that anemonin volatilizes. This is doubtless incorrect. Fehling states that heat softens and decomposes it, which is supported by our experiments; but we could not distill it. Heyer and Robert agree that if anemonin be fused, it is biting and burning to the taste, leaving a numbness on the tongue. We find that to even fuse anemonin is to partly dissociate it, one of the products being an intensely acrid, volatile, oily substance, which, in our opinion, produces the irritation.
If boiled with water, anemonin decomposes, a peppery, pungent, volatile oil distilling. This distillate is neutral, will not react with Fehling's solution, and is free from anemonin. It is a peppery, acrid liquid, and appears to depend for its properties upon the same volatile, oily substance that results from the dry destructive distillation of anemonin, showing rather positively that the destruction of anemonin under the action of prolonged boiling is similar to its decomposition by means of a quick, dry heat. [This might support the inference that anemonin exists in the plants, and decomposes to produce the volatile oil. We searched for it, and as previously stated, failed to find it.]
Anemonin dissolves freely in chloroform, sparingly in cold alcohol and benzol, and scarcely at all in carbon disulphide and sulphuric ether. Cold water dissolves only traces; boiling water dissolves it sparingly, the anemonin separating when the solution cools. Anemonin burns like camphor, with a bright flame. If heated, and then inflamed, it flashes. Pure anemonin does not evolve volatile products by age. We have an old specimen in our possession, however, which has assumed a partly insoluble condition (in chloroform), and become opaque.
Crystallized anemonin rapidly reduces Fehling's test solution for glucose. We can find no record of this fact in the authorities at our command, and we do not recall a volatile glucoside. This non-volatile character of the substances that conform to the glucosidal reactions, would also seem to support the inference that anemonin is a product and not an educt. The experiments made by us (as before stated), in an endeavor to obtain anemonin from fresh plants that yield it by distillation, were failures. Suitable solvents of anemonin did not extract from the plant any substance that would react with the glucosidal reagents. In connection with this point, we will say that specimens of pure crystallized anemonin prepared by us were examined by Prof. Virgil Coblentz, who also reports that pure anemonin is permanent; and we quote from his letter as follows:
"Different glucosidal reagents were tried upon this compound, resulting with immediate reduction of Fehling's and Schsse's (Ag (ic) Cl, KI, KOH) reagents. Böttger's, Schmidt's and Knapp's (Hg CN and Na OH) were only reduced after considerable boiling. Probably the slowness of reduction in these latter reagents was due to the insolubility of anemonin, while the rapidity of Fehling's might be on account of the solubility of the compound in the copper solution, as is the case with some organic compounds."
Dr. Fred. Hoffmann informs us that anemonin is still classed among the camphor-like bodies. [See also Pharmaceutische Rundschau, May, 1884, p. 98. One of, if not the, first names applied to this body (Störk, 1771), was Pulsatilla camphor.] It is certainly a very interesting substance to the theoretical chemist, and a valuable contribution will be the systematic study of the products of the distillation of Ranunculus, and of the results of their subsequent reactions and decompositions. Our study of the literature and the experiments we have made, assure us that these reactions are quite intricate, and that under certain conditions products arise that maybe absent under others. [1st. If a chloroformic solution of the substances obtained from Ranunculus abortivus be exposed to the air, the residue crystallizes, turns slowly yellow, and becomes strongly acid. 2d. If it be securely sealed, an amorphous, pure white magma of anemonic acid is deposited; the solution still (eight weeks) contains anemonin and the acrid oil, and is acid to litmus. 3d. Upon mixing alcohol with the chloroformic solution, by spontaneous evaporation a tough, leathery, yellow, extremely acid mixture results, destitute of pungency.] These facts have doubtless led to the differences between some of the statements that have been made by those who certainly were careful investigators. [We wish to call the reader's attention to the fact that our work, as recorded in July, pp. 60 to 64 inclusive, is not altogether supported by continued investigations in this number of the book. We find that a pure chloroformic solution of the principles obtained from the distillate has certainly become less pungent, and it is not unreasonable to suppose that greater time will note a complete destruction of this acrid principle. The liquid still contains an abundance of anemonin, a heavy and increasing deposit of white anemonic acid, and, when filtered, is of strong reaction to litmus, showing that the changes are somewhat complex. Time only can enable us to verify the supposition that the acrid principle will entirely disappear, to be replaced by the substances we have named, and thus practically support Erdmann. It will also be necessary to obtain fresh herb (next season), and estimate the proportions of these bodies and their compositions. At this writing we must say that, considering the unstable nature of the products of the distillation of this plant, and the uncertain and variable results of the subsequent changes, it is not to be wondered that investigators have disagreed. In the Addenda to Drugs and Medicines of North America, we shall continue the subject, but another season must pass before material can be obtained. It must not be forgotten that we are working with Ranunculus abortivus, a plant that others have not employed.]
Crystalline Form.—Anemonin crystallizes readily, sometimes in long needles, and again, from the same solution, in short, imperfect prisms. (See Fig. 24, p. 61.) In either case the faces are broken. Upon magnifying the crystals, it is found that few are developed sufficiently to allow of classification. It is now generally accepted that they belong to the right prismatic system.
ANEMONIC ACID.—C15H14O7 (Fehling). This formula would indicate that anemonin might change to anemonic acid by simply the taking up of a molecule of water, thus: C15H12O6 4 H2O = C15H14O7. That anemonic acid can result from the action of heat on moist crude anemonin is evident (p. 63, 4th), but we doubt if the change is as simple as the above equation would suggest. The formulae should be verified.
Preparation.—Expose to the atmosphere the chloroformic solution of the substances obtained from the distilled water of the acrid species of Ranunculus, occasionally adding a little water. Decompositions follow, and after some weeks a tough glutinous leathery substance remains. This contains impure anemonic acid, and is to be washed with chloroform (to separate anemonin), and then repeatedly boiled with alcohol to separate associated products, one of which is an acid.
2d. Allow a chloroformic solution, as named above, to stand in a sealed vial. Gradually a pure white substance separates, and will continue to deposit for some time. [Limit of time not yet determined by our experiments; and we have found no record from others.] The chloroform becomes of acid reaction to litmus. [This liquid still contains an abundance of anemonin, and after two months' time appears to be less pungent. This would seem to support the theory that the pungent principle, by dissociation or reaction on other bodies, might produce anemonin and anemonic acid (see Wittstein's Organic Constituents of Plants, p. 13).]
The white precipitate, if washed with chloroform and then repeatedly treated with boiling alcohol, is the substance named anemonic (not anemoninic) acid.
Properties.—Anemonic acid is white, tasteless (slightly astringent before drying), odorless, of acid reaction to litmus if moist, non-crystalline, and insoluble in all menstruums tested by us excepting those that decompose it. It is insoluble in any of the following, either cold or boiling, viz.: water, anhydrous alcohol, chloroform, carbon disulphide, sulphuric ether, benzol, and spirit of nitrous ether (5 per cent.). When dried and chewed, it is glutinous, reminding of tapioca.
Rabenhorst states that anemonic acid unites with bases to form salts; Heyer, that it dissolves in liquor potassa; Schwarz, that it colors alkaline solutions yellow, a yellow powder remaining. According to our experiments, this substance dissolves but little in alkaline liquids, but changes, in accordance to its purity, to an orange or red color, and swells considerably, becoming transparent and gelatinous. This gelatinous material, if formed of fresh, pure, white anemonic acid, is so nearly transparent that it is sometimes necessary to decant the solution before it can be observed; then it will be found an undissolved jelly, occupying many times the bulk of the anemonic acid employed. If the anemonic acid is old, this jelly forms more slowly, and is not so transparent; if it is fresh, and a very dilute solution of the alkali is used, the result will be a mucilaginous liquid. [In connection with this subject, we will say that Prof. Virgil Coblentz, to whom we sent a portion of purified anemonic acid, reports that it combines with alkalies, but that it is a very weak acid. He formed a lead and a soda salt, but the acid was nearly inactive.]
Rabenhorst states that nitric acid turns anemonic acid yellow, and then quietly dissolves it; and that this solution deposits flocculent matter upon the addition of water. Our experiments agree; and this precipitate we find to dissolve in alcohol or alkaline liquids.
Heyer states that sulphuric acid blackens it; and this is also supported by us, especially if the sulphuric acid is heated.
When heated in a tube, anemonic acid decomposes, a liquid condensing of a peppery taste. Vapor of this liquid is very irritating to the eyes. A carbonaceous mass remains.
Summary.—Anemonic acid is an amorphous body, formed by an undetermined reaction between certain volatile products of the distillation of fresh Ranunculus or of other plants that yield anemonin. The circumstances which give rise to it are such as yet demand considerable investigation.
It is a substance of feeble acid properties, but if fresh it will neutralize alkalies and form gelatinous substances which, in our experience, do not freely dissolve, but the jelly-like product is so nearly transparent as to often lead to the inference that it has formed a solution.
Anemonic acid is heavier than water. Many writers now seem to question the position of this substance; and Prof. Dragendorff, in his "Plant Analysis (1884), omits it entirely.
ANEMONINIC ACID.—Löwig and Weidman boiled anemonin with excess of baryta water, and, as a decomposition product, obtained red flakes of a substance containing barium. This they named anemoninate of barium, calling the acid which gave rise to it anemoninic acid. Prof. Fehling afterward investigated this substance, and demonstrated that this precipitate only amounted to 7-10 the anemonin employed; and therefore "the acid can not be formed from anemonin by simply assumption of water."
There is no doubt that one of the substances produced under these conditions is of acid reaction, but chemists must have a clearer insight into the decomposition products of anemonin than at present, before a systematic connection can be formulated between anemonin and this acid. Acid products arise when anemonin decomposes under several conditions, but the explanation off the molecular changes has not yet been demonstrated.
ANEMONOL (Oil of Anemone).—This is familiar as the acrid principle, already often mentioned by us. Some writers have spoken of anemonin as being acrid, and we accepted that view when we employed the term on page 22, but now find that this oil of anemone is the principal acrid substance.
Preparation.—Place the ethereal liquid (obtained in preparing anemonin, p. 64) in a shallow vessel, and evaporate the ether by means of a current of cold air. When the odor of ether is no longer apparent, anemonol remains, but evaporates quickly by exposure.
Properties.—Anemonol is exceedingly pungent and irritating. The vapor will stifle a person who carelessly inhales it, and will inflame the eyes, and even close them, It reminds us of volatile oil of mustard, but, according to Erdmann, is free from sulphur. Those who experiment with oil of anemone in considerable amounts, must exercise care and avoid exposure. Some of the oil was accidentally spilled on the skin of the ball of the writer's thumb. It produced a deep inflammation, and in two days blistered the point of contact as effectually as could have followed the application of a hot iron.
In diluted form, it produced watery blisters when sprinkled over the skin. This is the oil recorded by Heyer, Schwarz, Müller, Erdmann, and noticed by others, and has been obtained by the distillation of Anemone Pulsatilla, Anemone nemorosa, Ranunculus Flammula, Ranunculus bulbosus, and Ranunculus sceleratus. It is recorded as the substance that decomposes under certain conditions to form anemonin and anemonic acid.
OIL NO. 2.—This seems to have escaped the notice of others. It is not as volatile as anemonol. It is obtained in small amount by cautiously evaporating anemonol from crystals of anemonin, as obtained from the crude chloroformic solution. After the anemonol has vaporized, this (NO. 2) remains, and will adhere to the crude crystals of anemonin for days, and even weeks.
This second oil has a pleasant odor and a sharp taste. It exists in very small amount, unless it be that the intense pungency of the other oil (anemonol) overcomes it.
The second oil of anemone seems to be a product of chemical action after or during the condensation of the distilled water of the plant. At any rate, we failed to perceive it any part of the plant, or to obtain it direct from the herb.
Summary.—In conclusion, we sum the entire matter up as follows:
The peculiar acrid principle of many plants of the Ranunculaceae is a volatile oil. This oil preëxists in the plant.
Anemonin is a crystalline product of the distillation of the plants with water. It is not acrid. As associated with the other substances obtained from the distillate, it will decompose upon exposure, especially if moist, several undetermined substances resulting from the reactions. One of these is a fragrant volatile oil; another a soluble acid; a third, the substance recognized as anemonic acid; and the probabilities are that other bodies arise. The connections between these various substances, and the changes which take place in their formation, are still obscure, and we now hesitate to do more than present the results of the work of others as recorded in the preceding pages, and add thereto our own experiences; and in thus temporarily closing the subject, we regret its very incomplete condition. [We have arranged with a prominent worker in organic analysis, and by supplying him with materials of undoubted purity, shall enable him to enter systematically into the subject, but must await the coming season to obtain the fresh plant. The results will be announced in the Addenda to Drugs and Medicines of North America. It is certainly due the reader that we should state that, by an explosion and fire in our laboratory, the systematic work on this subject was interrupted, all the materials of this line of investigation being burned. Had we been permitted to follow the series to the end, we feel that more light would have been furnished on several obscure points.]
PHARMACOPOEIAL HISTORY.—The second edition of the United States Pharmacopoeia (1830, Philadelphia edition), recognized Ranunculus bulbosus in its secondary list. This position was retained through four revisions, the plant being discarded in 1880.
The New York edition of the Pharmacopoeia (1830) refused Ranunculus a position, and it would have been more creditable had others been as conservative. As it is, we find that for more than half a century this plant has been a member of the Materia Medica, and recognized by the highest authority in American medicine, but has never, during all of this time, received the support of a single eminent practitioner of the medical profession that recognizes the Pharmacopoeia. [Our remarks under Medical History and Properties do not refer to the Homoeopathic section of the medical profession. Ranunculus is recognized by them, as is shown by Prof. Hale's paper in this work.] There is no record to show that it was used by the regular medical profession at the time it became officinal, and there was no excuse for carrying it sixty years.
- Part Used.—U. S. P., 1830 (Philadelphia), "The plant Ranunculus bulbosus."
- 1840, 1850, 1860, "The cormus and herb of Ranunculus bulbosus."
- 1870, "The corm and herb of Ranunculus bulbosus."
PREPARATIONS.—There are no preparations of Ranunculus in use excepting those of Homoeopathic physicians. We therefore extract from the Pharmacopoea Homoeopathica Polyglotta as follows:
Essence of Ranunculus bulbosus.—"The fresh flowering plant is gathered in the month of June, separated from the bulbs, and the juice pressed out. The bulbs are then pounded with some strong alcohol into a jelly, and likewise pressed out. The liquids, thus obtained, are mixed with equal parts by weight of strong alcohol. To the remains of the bulb-jelly add two parts of strong alcohol, macerate for three days, and press as before. The liquor, thus obtained, is mixed with the former liquids, macerated for eight days, and filtered."
MEDICAL HISTORY.—The several acrid species of Ranunculus have been mentioned in European medicine from an early day. They were more conspicuous members of the Materia Medica in times long past than at any recent period. Indeed, this seems natural, for the semi-barbarous treatment of former times induced physicians to eagerly accept a substance that could torture the patient like the acrid Ranunculus can, when heroically applied. Hence we find (1710) that Salmon's English Herbal devotes page after page to the plants of the Crowfoot tribe, enumerating and figuring eight different species. Following this work, we find that other European authors mention it, but with less respect; and at the present day the plant is on record as a vesicant and an acrid poison, unsupported by the friendship of a single prominent practitioner outside of the Homoeopathic section, which would not use it in this manner. Prominent medical writers have given it a position, always seemingly under protest; and in this way we find it mentioned by modern authorities.
MEDICAL USES.—In early English medicine the acrid "Crowfoot" was recommended for a multitude of disorders. Salmon (1710) scarcely excepted an ailment to which the flesh is heir; and in reviewing his paper we are puzzled to discover the necessity for any other medicinal agent. Of Crowfoot, he writes:
"The Liquid Juice.—It is sharp and biting, good to bathe gently those parts of the skin which are effected with Scurvy, Morphew, Leprosie, Freckles, Spots, Yellowness, Roughness, etc. It is good also to drive away Scabs and Itch."
"The Essence.—It is good to waste away and consume Warts, Corns, hard Scabs of the Skin, Ruggedness of the Nails, and other like deformities of the Cutis. The head being washed with it, it kills worms at the roots of the hair, which eat the same and cause it to fall off. Neither juice nor essence, by reason of their violence, are ever used inwardly."
This strain is continued to ten times the amount of space quoted by us, and, included in the list, we find that crowfoot will cure ague, toothache, tumors, scrofula, itch, bleeding piles; is a diuretic, an emetic; will dissolve stone in the bladder; and finally, that it is a parturient. Meyrick (1790) quotes from Hill, that "all parts of these plants are exceeding acrid, and if bruised, act as a caustic." Cullen's Materia Medica (1802) classes the Ranunculus among the diuretics, stating, however, that "they have, as such, been hardly employed in practice: and that for the same reason I have given with respect to the Arum, that we have not yet learned how they can be introduced in such quantity into the stomach as to become powerful in the kidneys." Hand (1820), in his House Surgeon, states that crowfoot "produces a deep and thorough blister. Good where a lasting blister is wanted." ["Lasting blister" is a good term. The writer applied the Ranunculus to a portion of his arm. It irritated, and caused watery blisters to form over several times the surface exposed. The blisters lasted for days, the irritation for weeks. The cuticle peeled off, and it was a decided success, if success can be valued in proportion to the "lasting" properties.] The Supplement to the Pharmacopoeia (London, 1821) ascribes medical properties to the several acrid species of Ranunculus, stating that Ranunculus bulbosus will poison rats. And in 1865, we see it recorded in the Pharmaceutical Journal and Transactions that a child in England perished after eating the blossoms of crowfoot. Chapman's Therapeutics (1824) mentions crowfoot as a vesicant, saying, "and most promptly and powerfully does it operate." Chapman admits, however, that he has only seen it used to blister horses, and cautions his readers against the free use of crowfoot, in the following language: "By many means we raise a blister, and by some in much less time than with cantharides, yet there is none which precisely imitates their mode of action, or will do equal good in the cure of disease. Considering its (Ranunculus) great activity, I am inclined to suspect that we might make some beneficial application of it, though on this account alone it should not supercede the animal vesicatories." In 1858, Prof. Clarus, of Leipsic, instituted a line of physiological experiments with the acrid species of Ranunculus. He decided that anemonin was a narcotic, and the volatile oil an acrid irritant. Krapf, of Vienna, from a series of experiments with the acrid species of Ranunculus, found that no antidote experimented with could reduce the inflammation; and, indeed, that while water gave the best results, vinegar, honey, sugar, spirits, diluted mineral acids and solutions of alkalies increased the acrimony. In our recent experience, both our hands and mouth having been blistered, we exhausted the probable antidotes without relief.
Summary.—In the olden time the different acrid species of Ranunculus were used rather freely in medicine. As the practice of medicine inclined towards a humane system, physicians gradually substituted less virulent remedies; and in modern times we find that few who give large doses care to use such agents as the Ranunculus plants, even externally. They produce painful, sometimes deep ulcers, and blister some persons very quickly. However, this action is not always certain; and sometimes we find subjects who seem not to blister under their influence, the result being a deep inflammation. The ulcers are slow to heal. In the opinion of the writer, a physician would hardly at this day be excused for resorting to such a barbarous agent, if used in concentrated form.
HOMOEOPATHIC USES OF RANUNCULUS BULBOSUS.—(Written for this publication by Prof. E. M. Hale.)
This species appears, from the experiments and practice of our school, to have a decided irritant action on the brain and spinal cord. It has been successfully used for neuralgia of the head, with vertigo and confusion of mind. A study of its symptomatology shows it to cause conditions which have been termed neuralgic rheumatism. Many of the pains closely resemble those of myalgia, or what has been known as muscular rheumatism. It appears to me to be a close analogue of cimicifuga. The latter, however, seems to affect by preference the large muscles, and particularly the belly of the muscles; but the former seems to attack the small, thin muscles, as those of the head and thorax. Both have an affinity for the sheaths of the nerves, rather than the axis. Pure neuralgia does not affect the sheaths of nerves, but the nerve itself; but rheumatic neuralgia affects the sheaths.
I have used it very successfully in rheumatic headache, aggravated by or brought on by change of weather from warm to cold and damp. These headaches closely resemble neuralgia, but will not be relieved by the purely neuralgic remedies. In rheumatic affections of the eyes it has been curative. It is an important remedy in rheumatic affections of the muscles of the abdomen, thorax and back. Nearly all the pains which indicate Ranunculus are stitching, with soreness, aggravated by motion. In the abdomen these pains are often mistaken for colic. When affecting the thorax, they are often mistaken for pleurisy. It is one of our best remedies in pleurodynia, intercostal rheumatism and myalgia. The thoracic pains extend to the back, and take the form of lumbago. All the muscles of the trunk seem to be affected with soreness, stiffness, stitches, etc., especially when first moving after sitting or lying. These symptoms will be recognized as those which are very common in a class of people exposed to changes of temperature, and who have an acquired or inherited rheumatic tendency.
The experiments with Ranunculus bulbosus have not been thorough enough to warrant us in recommending it for true spinal irritation, but, judging from analogy, we believe it to be capable of acting as an irritant to the spinal meninges. Most cases of so-called spinal irritation are either rheumatic or the result of anemia and debility. In the former Ranunculus will be specific; the latter will be most successfully treated with iron and hydrastis.
Ranunculus sceleratus and acris have an almost identical sphere of action, and are indicated by very similar symptoms.
The action of various species of Ranunculus on the skin is local, and caused by an acrid irritant in the juice of the plants. There is no proof that the internal administration of the drug has caused any eruptions; but the blood seems to be poisoned by the absorption of the secretions. The local affection takes the form of vesicles, blisters, or bullae, and resembles pemphigus and some varieties of erysipelas.
Mezereum (Daphne Mezereum) has a similar local action, but in addition has actually caused, when taken internally, vesicles and bullae, attended by violent stitching pains, such as precedes herper zoster. Ranunculus may be capable of similar action. If so, it will prove a remedy for herpes.
The secondary effects of this drug on the skin take the form of boils and obstinate ulcers, and even cutaneous abscesses. We observe the same effects from Rhus Toxicodendron.
In practice Ranunculus bulbosus and sceleratus have been used with good results (internally) for pemphigus, herpes on the hands and fingers.
Pharmaceutical and Medical References to Ranunculus and Anemonin.
- 1710.—Salmon's English Herbal, pp. 243 to 253.
- 1762.—Histoire des Plantes de L'Europe, Part 1st, pp. 292 to 296.
- 1787.—Materia Medica Americana, Schoepf, p. 93.
- 1790.—Meyrick's Family Herbal, p. 128.
- 1801.—Collections for a Vegetable Materia Medica, B. S. Barton, p. 52.
- 1802.—A Treatise of the Materia Medica, Cullen, p. 312.
- 1818.—The American Dispensatory, Coxe, p. 479 (and other editions).
- 1820.—The House Surgeon and Physician, Hand, p. 244.
- 1821.—Supplement to the Pharmacopoeia, London, p. 150.
- 1824.—Elements of Therapeutics and Materia Medica, Chapman, 1824, p. 105 (and other editions).
- 1825.—Hooper's Medical Dictionary, p. 1018 (and other editions).
- 1826.—A Materia Medica of the United States, Zollickoffer, p. 220, 224,
- 1827.—The Medical Companion; or, Family Physician, Ewell, p. 656.
- 1827.—Outlines of Lectures on Materia Medica and Botany, W. P. C. Barton, Vol. II., p. 253.
- 1829.—A Manual of Materia Medica and Pharmacy, Edwards and Vavasseur, p. 75 (American reprint).
- 1830.—United States Pharmacopoeia, Philadelphia edition, p. 36.
- 1830.—Medical Flora of the United States, Rafinesque, p. 72.
- 1830.—The Botanic Physician, Smith, p. 448.
- 1833.—United States Dispensatory (1st edition), p. 525 (and other editions).
- 1835.—The Thomsonian Recorder, p. 152.
- 1836.—General Therapeutics, Dunglison, p. 376.
- 1840.—United States Pharmacopoeia, p. 47.
- 1840.—Pharmacopée Universelle, Jourdan, p. 363.
- 1847.—Medical Botany, Griffith, p. 83.
- 1848.—A Catalogue of the Medicinal Plants of New York, Lee, p. 4.
- 1848.—Christison and Griffith's Dispensatory, p. 797.
- 1848.—Mayne's Dispensatory, p. 179 (American reprint).
- 1849.—Indigenous Medicinal Plants of South Carolina, Porcher, p. 685.
- 1850.—United States Pharmacopoeia, p. 53.
- 1850.—General Therapeutics and Materia Medica, Dunglison, p. 260 (and other editions).
- 1851.—Medicines: their Uses and Mode of Administration, Neligan, p. 206.
- 1852.—A Dictionary of Medical Science, Dunglison, p. 741.
- 1852.—American Eclectic Dispensatory, King & Newton, p. 213 (and subsequent editions).
- 1854.—Elements of Materia Medica and Therapeutics, Pereira, p. 1080.
- 1859.—American Journal of Pharmacy, p. 440.
- 1859.—Eclectic Medical Journal, Cincinnati, p. 414.
- 1860.—United States Pharmacopoeia, p. 61.
- 1860.—Druggist's Circular, p. 59.
- 1861.—American Journal of Pharmacy, p. 528.
- 1864.—Hand-book of Chemistry, Vol. XVI., Gmelin, p. 265.
- 1865.—Pharmaceutical Journal and Transactions, p. 38.
- 1865.—American Eclectic Materia Medica, Hollemback, p. 316.
- 1866.—Druggist's Circular, p. 89.
- 1866.—American Eclectic Materia Medica and Therapeutics, Jones & Scudder, p. 580.
- 1870.—United States Pharmacopoeia, p. 60.
- 1870.—Eclectic Medical Journal, Cincinnati, p. 440.
- 1871.—Journal of Materia Medica, Tilden & Co., p. 144.
- 1872.—Pharmacopoea Homoeopathica Polyglotta, PP. 117, 214.
- 1874.—A Treatise on Pharmacy, Parish, pp. 420, 520 (and other editions).
- 1878.—Organic Constituents of Plants, Wittstein, p. 13.
- 1879.—The National Dispensatory, pp. 118x, 1182.
- 1882.—The American Practice of Medicine, Goss, p. 332.
- 1884.—Plant Analysis, Dragendorff, (Greenish), p. 109.
- 1884.—Companion to the United States Pharmacopoeia, Oldberg and Wall, p. 825.
Drugs and Medicines of North America, 1884-1887, was written by John Uri Lloyd and Curtis G. Lloyd.

