 The rhizome of Curcuma longa, Linné (Curcuma rotunda, Linné; Amomum Curcuma, Jacquin).
The rhizome of Curcuma longa, Linné (Curcuma rotunda, Linné; Amomum Curcuma, Jacquin).
Nat. Ord.—Zingiberaceae.
COMMON NAMES: Turmeric (long and round), Curcuma.
ILLUSTRATION: Bentley and Trimen, Med. Plants, 269.
Botanical Source.—This is a perennial plant, with the roots or tubers oblong, palmate, and deep-orange inside. The root-leaves are about 2 feet long, lanceolate, long-petioled, tapering at each end, smooth, and of a uniform. green color. The petioles are sheathing. The spike is erect, central, oblong, and green. Its flowers are dull yellow, arranged 3 or 5 together, surrounded by bracteolae (L.).
History.—Turmeric is indigenous to several parts of southern and eastern Asia, and is extensively cultivated in China, Hindustan, and other countries, where it is propagated from cuttings of the root. Several varieties are found in commerce, their names being derived from the countries producing them. Thus we have Chinese, Madras, Bengal, Java, and Cochin turmerics. The latter is thought to be derived from another species than Curcuma longa, Linné, and though never used as turmeric in Cochin, the natives obtain from it a variety of arrow-root. The other varieties differ somewhat, though in general not enough to be noticed by inexperienced persons. The Chinese turmeric is generally preferred. Java turmeric is the least valuable, and is the product of Curcuma longa, var. minor, Hasskarl. Curcuma is chiefly used as a condiment and for dyeing; the Bengal variety, which is of a deeper color, being generally preferred for the latter purpose. While the foregoing varieties of curcuma are recognized, the drug is generally distinguished in commerce by the terms long and round turmeric. The latter is the central or primary rhizome of the plant, while the former consists of the lateral or secondary rhizomes. Turmeric is largely used in the preparation of Turmeric Test Paper, and also for coloring table mustard and some other food products.
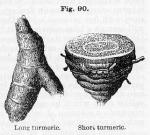
Description.—Long turmeric (curcuma longa), when dry, is in slightly curved, subcylindrical, or oblong tubers, about 1 to 3 inches in length, and from ¼ to ½ inch in diameter, often pointed or tapering at one end, yellowish externally, with transverse, parallel rings, internally deep-yellow or reddish-brown, marked with shining points (due to immersion in scalding water in order to facilitate the drying process), dense, solid, having a short, granular fracture, and forming a lemon-yellow powder. Round turmeric (curcuma rotunda), differs in being ovate, pear-shaped, or subspherical, occasionally pointed and crowned with leaf remains at the upper end. They are from 1 ½ to 2 inches long, and from ¾ to 1 ½ inches in diameter. Turmeric has a peculiar, somewhat fragrant odor, and a bitterish, slightly acrid taste, like that of ginger, exciting a moderate degree of warmth in the mouth, and communicates a yellow color to the saliva. It yields its properties to water or alcohol.
Chemical Composition.—The yellow coloring matter, curcumin, was first obtained (impure) by Vogel and Pelletier; more recently it was prepared in pure and crystalline form by Daube (1870), and contemporaneously by Ivanow-Gajewsky. It was further studied and some derivatives of it prepared by C. Loring Jackson and A. G. Menke, in 1882-83, who determined the formula C14H14O4. These chemists obtained it from the root by first removing the fatty and volatile oil by means of ligroin, which removed 11 per cent of the weight of the root, then abstracting curcumin (contaminated with resins), by means of ether, and repeatedly crystallizing from alcohol until the melting point became constant, viz.: 178° C. (352.4° F.). The yield was 0.3 per cent. As thus obtained, curcumin crystallizes in amber-yellow prisms, appearing orange-yellow in reflected light, insoluble in water and diluted acids, sparingly soluble (1 in 2000) in benzene (Daube's solvent, which refuses to dissolve the resins); easily soluble in ether, chloroform, and alcohol; also in diluted alkaline solutions with a striking red-brown, fluorescent color. It is partly soluble, with crimson color, in concentrated acid solutions. Non-alkaline solutions exhibit green fluorescence. Chemically, curcumin is a substituted aromatic oxyacid, yielding vanillin with weak oxidizers, and protocatechuic acid when fused with caustic potash.
Filtering paper saturated with an alcoholic solution of curcumin and dried, reacts like turmeric tincture (see below) towards acids, alkalies, and boric acid. It turns red-brown with alkalies which color becomes violet upon drying; the yellow color is restored with acids. When the paper is dipped into solution of boric acid, it turns orange-red (upon drying), and remains so even when it is afterward moistened with free mineral acid. Paper that has been turned to orange by boric acid will assume a blue color when it is moistened with diluted alkali. Schlumberger (1866) obtained crystalline rosocyanin by heating an alcoholic solution of curcumin and boric acid with mineral acids and then allowing the blood-red solution to cool. It is insoluble in water, but soluble in alcohol, this solution turning blue upon the addition of an alkali.
A red compound of curcumin with boric acid was obtained in the form of a precipitate by the same author, by adding water to a mixture of alcoholic curcumin and boric acid; boiling water removed therefrom all the boric acid, leaving a yellow resin which he called pseudo-curcumin. Strong sulphuric acid forms with curcumin a solution of crimson color, which color disappears upon dilution with water, a precipitate of yellow flakes resulting.
About 1 per cent of an acrid volatile oil is present in curcuma root, one constituent of which, called turmerol, was isolated, in 1883, by C. L. Jackson and A. G. Menke, the boiling point being about 193° C. (379.4° F.). It has a pleasant aromatic odor, is lighter than water, and is oxidized by means of potassium permanganate to terephthalic acid (C6H4[COOH]2). The presence of alkaloids in curcuma root was indicated by Ivanow-Gajewsky and by J. Cook. Kachler, in 1870, obtained considerable amounts of acid potassium oxalate from an aqueous infusion of the root.
TURMERIC TINCTURE and TURMERIC PAPER.—Turmeric is much employed as a test for alkalies, which render it reddish or brownish; white bibulous paper or paper not sized, is brushed over with the tincture or decoction, or dipped into one of them, and dried in a neutral atmosphere (see Reagents and Test Solutions of U. S. P., No. 59).
Action, Medical Uses, and Dosage.—Turmeric is a mild, aromatic stimulant, but is seldom used in this country, except to color ointments and pharmaceutical mixtures.
Related Product.—Murraya Koenigii (Nat. Ord.—Rutaceae), Curry leaves. The leaves of this tree, which grows wild in the mountains of the western coast of India, are quite extensively used as a condiment. They enter into the mixture known as Curry powder, largely used in pickling and cooking. In India the leaves are regarded as tonic and stomachic, and are given raw in dysentery and applied externally in skin eruptions. The Hindus employ an infusion of the leaves to check vomiting (Ainslie). The root is stimulant, and is in repute as a remedy for the bites of venomous animals (Dymock, Mat. Med. of Western India). An essential oil, two resins, and a bitter glucosid, koenigin, were obtained from the leaves. Simabolee oil is obtained from the seeds. The white, sweet-odored flowers of Murraya exotica (cultivated), yielded (De Vrij) the glucosid murrayin, (C18H22O10) (Dymock).

