Continued from previous page.
Muriate or Hydrochlorate of Berberine - Mono-Berberine Sulphate (Bisulphate of Berberine) - Di-Berberine Sulphate (Normal Sulphate of Berberine)- Phosphate of Berberine
MURIATE OR HYDROCHLORATE OF BERBERINE, C20H17NO4.HCl. Crystallized, C20H17NO4.HCl+2H2O.—The introduction into American medicine of the salts of berberine was an outgrowth of the introduction of the "concentrations" of early Eclecticism, and intimately connected with it. Therefore, we shall introduce muriate of berberine by the historical connection.
The preparation of podophyllin (Resin of Podophyllum, U. S. P.) in 1847 [See Eclectic Medical Journal, Cincinnati, January, 1849, p. 1, and compare statement of Wm. S. Merrell in American Journal of Pharmacy, 1862, p. 496.] led to the preparation by similar processes of other materials from Cimicifuga racemosa, Veronica virginica, Sanguinaria canadensis, Cypripedium pubescens, and Hydrastis canadensis. [See Eclectic Medical Journal, Cincinnati, January, 1849, p. 1, and compare statement of Wm. S. Merrell in American Journal of Pharmacy, 1862, p. 496.] All of these substances were first made after the method employed in preparing podophyllin, by simply evaporating the alcohol from a tincture of the respective drug, and then pouring the creamy liquid into cold water. The precipitate, if it were capable of drying, was powdered and sold in that form; but if it was an oleoresin, it was distinguished as a "soft concentration." In an advertisement before us of August, 1852, we note, under the heading of "Concentrations":
- "Powders.—Podophyllin, Leptandrin, Macrotin, Myricin, Sanguinarin, and Hydrastin. [These substances were mentioned by the late Wm. S. Merrell in the Eclectic Medical Journal, Cincinnati, July, 1852, p. 297. We have found no earlier record outside of the discovery of podophyllin by Prof. John King some years previously.]
- "Soft Concentrations.—Ptelein, Apocynin, Eupatorin, Asclepedin." [Afterward the more appropriate term oleoresin was given to the "soft concentrations."]
Thus it happened that because podophyllum chanced to yield an active medicinal agent by this method, it was accepted that other similar substances would necessarily prove to be valuable, and Hydrastis was included. However, it was soon found that the precipitate, so-called Hydrastin, neither retained the sensible nor medicinal properties of Hydrastis. It is true that it had a yellow color, and was bitter; but the overlying liquid beyond a doubt contained the valuable constituents of the drug. These obvious facts led to experiments having for their object the separation of the real characteristic principles. After repeated trials, it was found that the addition of muriatic acid to the supernatant liquid (from which the so-called hydrastin had been precipitated) produced a brilliant yellow precipitate, that was very bitter, and seemed to possess the greater part of the sensible properties of the rhizome of Hydrastis. This was introduced into medicine as hydrastin neutral, to distinguish it from the former resinous substance known as hydrastin. [See the first edition of the Eclectic Dispensatory, King & Newton, 1852, p. 214.]
The introduction of this second substance inaugurated a confusion in nomenclature that, with the products of Hydrastis, has remained even to the present day. We find the two materials recorded in the prices current of the three principal manufacturers of those times, under three names, to-wit:
| MAKER. | RESINOUS PRECIPITATE. | MURIATIC ACID PRECIPITATE. |
| No. 1. | Hydrastin. | Muriate of Hydrastin. |
| No. 2. | Hydrastin. | Hydrastin Neutral. |
| No. 3. | Hydrastin. | Hydrastine. |
Thus it is that muriate of berberine was the first salt of the alkaloid used in American medicine, and was at first known by three names. Indeed, it was eventually known by four, because in a short time the resinous precipitate called hydrastin dropped from use, and the term hydrastin became affixed to this substance, muriate of berberine.
Eventually the name hydrastine neutral [In those days this term was not so inappropriate. There was no known salt of muriatic acid and an alkaloid. of a practically insoluble nature, and consequently the salt was first thought to be an inactive chemical body which was thrown out of solution by the acid. We also call attention to the fact that Buchner and Herberger obtained the same substance, and regarded it as a weak acid or neutral principle.] was lost, and this muriate of berberine remained, for a considerable time, in American medicine under the names hydrastin, hydrastine, and muriate of hydrastine. [Afterward another link was added to this unfortunate chain of names by the introduction of "Principles Combined hydrastin." This is a mixture of various substances, and is expected to represent all the peculiar constituents of hydrastis. It is now the only substance recognized simply as hydrastin or hydrastine, these names having, by common consent of manufacturers, been affixed to it exclusively.]
It will be seen from this review of the early history of the substances derived from Hydrastis canadensis, that the first definite principle introduced into American medicine was the muriate (hydrochlorate) of berberine. The reader will also note that this was in reality a salt of berberine, for (see our history of the alkaloid berberine) Mahla [Am. Journ. Science and Arts, Jan., 1862, and Am. Journ. Pharm., 1862, p. 141.] demonstrated their identity, and Mr. Wm. S. Merrell immediately accepted the statement of Mr. Mahla regarding the substance that he (Merrell) had sold as hydrastine neutral. In the Eclectic Medical Journal, April, 1862, (Mr. Mahla having made the statement in January, 1862,) Mr. Merrell writes: "The fine yellow powder which we have heretofore sold as hydrastine neutral proves to be a true muriate of the hydrastia." He afterwards, in the American Journal of Pharmacy, stated that the alkaloid known as hydrastine was identical with berberine. Thus it is that throughout the length and breadth of this country, hydrochlorate of berberine at the present day is recognized as muriate or hydrochlorate of hydrastine, and in this connection we refer the reader to our history of berberine.
The Alkaloidal Nature of Muriate of Berbetine.—It is generally accepted that the identification of this substance as obtained from hydrastis was first made by Mr. Mahla in 1862 (see p. 99), but in reality he only announced that it was the same as muriate of berberine. It had been placed under the name hydrastine muriate with the alkaloids ten years or more before that, and described in language so expressive, that the definition would be a fair one at the present day. In support of our view of this matter, we quote from "Positive Medical Agents," by Grover Coe, 1855 (written before 1854), as follows:
"Hydrastine. This is the alkaloidal principle of Hydrastis canadensis. Hydrastin is a resinoid which is obtained from Hydrastis canadensis. As the reader may wish to know why we name these distinct principles so nearly alike, it may not be improper to give the required information at this point. The resinoids and alkaloids, being clearly distinct, and yet often derived from the same plant, it has been thought best to give the generic name of the plant to the active or concentrated principles, ending them in "in" when the active principle is of a resinoid character; and in "ine" when of an alkaloid character; thus we have hydrastin, a resinoid; and hydrastine, an alkaloid."
It will be seen that, while Mr. Mahla announced the identity of berberine with this yellow alkaloid of hydrastis, he was not the first to find its alkaloidal nature as obtained from hydrastis. ( See page 98.) In considering the matter further, we note that the nomenclature then adapted agrees with that now recognized by scientists, the alkaloid terminating in ine.
Preparation of Muriate (Hydrochlorate) of Berberine.—This salt can be made by precipitating either an aqueous or an alcoholic percolate of hydrastis with an excess of hydrochloric acid. In each case considerable amounts of the impurities are thrown down with the crystalline magma, which can only be completely freed from these associations by repeated crystallizations from both boiling alcohol and boiling water. Therefore it is that we prefer to prepare muriate of berberine from the di-berberine sulphate (by which process no heat is necessary), for it is best to avoid an extended application of heat. If, however, the process adopted be that of the direct production of muriate of berberine from the percolate of hydrastis, a considerable excess of muriate acid is necessary to dissociate the natural combination in which berberine exists, and simply bringing the liquid to an acid reaction, will only throw down a portion of the alkaloid. [See our remarks under Hale's Third Alkaloid of hydrastis.]
We therefore introduce the following process, announced first by us in 1878: [Proceedings American Pharmaceutical Association, 1878. Also American Journal of Pharmacy, 1879, January, p. 11.]
Dissolve di-berberine sulphate [At that time we supposed this to be berberine.] (C20H17NO42 2H2SO4) in sixteen times its weight of distilled water, and cautiously add hydrochloric acid until in slight excess; drain the precipitate, wash it with distilled water until free from sulphate and muriate of ammonium, and then dry it by exposure to the atmosphere. If desired in crystalline form, dissolve it in boiling alcohol and permit the solution to cool. Hydrochlorate of berberine, made by precipitation, is an odorless, bright, lemon yellow, crystalline powder. When crystallized from hot alcohol by rapid cooling, and then dried, it is in the form of light-yellow, delicate, soft, silky needles, so fine in texture, that a mass of the salt is spongy and cotton-like to the touch. If the crystals are larger, the color is darker, and when of considerable size, they are of a deep orange. Figure 34 (next page) represents a 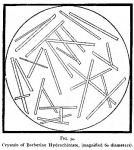 micro-drawing of this salt (prepared by us), drawn by Mr. Huck, under the supervision of Prof. Power, who writes: "Berberine hydrochlorate (C20H17NO4HCl+2H2O) was mounted in Canada balsam and magnified 60 diameters. The crystals have the form of distinct acicular prisms, and resemble very much in form the mono-berberine sulphate, but are relatively much larger."
micro-drawing of this salt (prepared by us), drawn by Mr. Huck, under the supervision of Prof. Power, who writes: "Berberine hydrochlorate (C20H17NO4HCl+2H2O) was mounted in Canada balsam and magnified 60 diameters. The crystals have the form of distinct acicular prisms, and resemble very much in form the mono-berberine sulphate, but are relatively much larger."
Nitric Acid, Action on Hydrochlorate of Berberine.—Fleitmann states that, when hydrochlorate of berberine is added to strong nitric acid, a dark-red solution is formed, and that the application of heat to this solution causes effervescence with liberation of nitric oxide (NO), and that when the nitric oxide escapes, the liquid changes to a lighter color. These statements are supported by our investigations, and we find that if a considerable proportion of hydrochlorate of berberine is used, and the heat continued until the solution changes from dark-red to orange, the liquid will be transparent and syrupy. The liquid which results, mixes with alcohol, hydrochloric acid, nitric acid and officinal ether, but not with chloroform or benzol. With ammonia-water, it forms a dark-red liquid, and this will mix with water. Sulphuric acid also forms with it a dark-red solution, but this quickly changes to orange, with the evolution of gas bubbles. When diluted with water, an abundance of a yellow precipitate (b) results, which, when dry, presents the following characteristics: This precipitate is very bitter pulverulent at ordinary temperatures, but falls into a brittle mass when heated. It dissolves in officinal alcohol and ether, (but not in concentrated ether), forming in both instances orange-colored liquids, which stain organic matters yellow. Solutions of the hydroxides of potassium, sodium and ammonium, dissolve it, dark-red liquids resulting, which mix with water and alcohol in all proportions. The foregoing reactions would lead to the inference that this substance might be picric acid, but it is distinguished by the following properties: It is insoluble in chloroform and benzol. Its solutions do not precipitate with solution of ammonio-sulphate of copper, hydroxide of potassium, nor with solution of cinchonine in diluted sulphuric acid; and it gives no reaction with cyanide of potassium. Fleitmann calls it a yellow, difficultly soluble wax. Mr. H. Weidel has studied the oxidation products of berberine, and described berberonic acid, formed by the action of nitric acid on berberine, which can not be identical with this body, as he describes it as colorless, glassy crystals. [We have access only to a summary of this paper, and therefore can not review it as we would like.]
Oxalic acid is another product of the action of hot nitric acid on hydrochlorate of berberine, and this is contained in the filtrate which passes when the precipitate (b) is separated. Both Henry and Buchner identified oxalic acid, and Buchner states that he obtained only oxalic acid as a result of the reaction. According to our experiments, the oxalic acid is in small amount, but if the proportions of the ingredients are varied, there might be a different result. In addition to the substances we have named, a yellow coloring matter results during the reaction between hot nitric acid and hydrochlorate of berberine, and this is soluble in water, alcohol, dilute acids and dilute alkalies. These decomposition products deserve further consideration.
Sulphuric Acid, Action on Hydrochlorate of Berberine.—Sulphuric acid dissolves hydrochlorate of berberine with production of a lemon-yellow liquid, which, when heated, darkens, changes to a greenish brown, and finally to dark brown. When the solution of hydrochlorate of berberine in cold sulphuric acid is permitted to stand, it changes to yellowish-brown. There is a difference in statements concerning this reaction, for according to Buchner, the resultant solution is greenish-yellow; Chevallier and Pellatan, red-brown; and Polex, violet-red. Our experiments were made with the perfectly pure salt.
Hydrochlorate of berberine is dissociated when boiled with an excess of dilute sulphuric acid, bi-sulphate of berberine and hydrochloric acid resulting. This was part of Fleitmann's process for making the alkaloid (see page 101) If the amount of berberine hydrochlorate be great, a considerable proportion of the resultant bi-sulphate remains undissolved in consequence of the slight solubility of the salt in dilute sulphuric acid, even if hot. Ammonia water dissolves it immediately, the solution conforming to all the reactions of di-berberine sulphate.
Fleitmann states that the "reddish yellow" solution of hydrochlorate of berberine turns pale yellow on the addition of dilute sulphuric acid, and that the mixture, after a time, deposits delicate, pale, reddish-yellow needles. According to our investigations, a cold saturated solution of hydrochlorate of berberine is greenish-yellow, instead of "reddish-yellow," which latter color indicates the presence of impurities. When acidulated with sulphuric acid, it deposits minute lemon-yellow (instead of reddish-yellow) crystals, which dissolve at once when the liquid is rendered alkaline with ammonia water, thus showing that cold sulphuric acid in excess will decompose muriate of berberine with the production of a sulphate.
Hydrochloric Acid, Action on Hydrochlorate of Berberine.—Cold hydrochloric acid dissolves only traces of hydrochlorate of berberine, but, more freely upon boiling, forming a lemon-yellow liquid. The salt is not decomposed, and it crystallizes when the boiling solution cools.
Acetic Acid, Action on Hydrochlorate of Berberine.—Cold glacial acetic acid dissolves small amounts of hydrochlorate of berberine, and freely when boiling. A crystalline deposit forms when the hot liquid cools.
Hydroxide of Ammonium, Action on Hydrochlorate of Berberine.—Cold ammonia water dissolves small amounts of hydrochlorate of berberine apparently without decomposition, for the solution has the light yellow color of a solution of hydrochlorate of berberine. When boiled with an excess of ammonia water, an orange-colored liquid results, and upon further boiling a slight brownish precipitate is thrown down. It is apparent that the heated ammonia first liberates a small amount of berberine, which dissolves with the orange color, and is then partly decomposed, with production of the brownish substance. Upon cooling such a solution, an abundance of yellow crystals results, which, according to our examination, contain hydrochloric acid, and conform to the reactions of hydrochlorate of berberine. Schaffner states that warm ammonia water forms a dark brown liquid with hydrochlorate of berberine, from which brown crystals are deposited upon cooling, a reaction we were unable to verify. Buchner and Schaffner supposed that ammonia combined with berberine under these circumstances, but we have not been successful in uniting them.
Hydroxide of Potassium or Sodium, Action on Hydrochlorate of Berberine.—Dilute boiling solutions of these alkalies dissolve hydrochlorate of berberine with liberation of berberine. This is shown by acidulating with sulphuric acid, whereby mono-berberine sulphate is produced. Concentrated hot solutions of these alkalies decompose the berberine, with production of a yellow resinous substance, almost insoluble in water. (See also decomposition products of berberine, p. 109). Hot dilute alcoholic solution of caustic potash acts like the aqueous solution of this alkali.
The carbonates of sodium and potassium, in dilute or concentrated solution, act like the alkalies.
Solubilities.—100 parts of distilled water, with one-eighth part of the undissolved salt, dissolved.204 parts of the hydrochlorate. Under the same conditions 100 parts of officinal alcohol dissolved.400 parts of the salt and 100 parts anhydrous alcohol dissolved.320 parts. It is practically insoluble in ether, chloroform or carbon disulphide.
Incompatibles.—The intense affinity that hot hydrochloric acid has for berberine renders this salt exceedingly stable, and, as we previous stated, led the discoverers to view it as a neutral body, or a weak acid. Consequently, it is not dissociated as easily as other alkaloidal salts. Boiling with excess of the mineral acids displaces the hydrochloric acid with the other. Solutions of the salts of silver decompose it immediately, and this is true of oxide and phosphate of silver, especially at high temperatures.
Hydrochlorate of berberine is mostly precipitated from aqueous solution by the addition of either hydrochloric or nitric acid, and largely, but less quickly, by sulphuric acid. Upon boiling with an excess of these acids, it is dissociated. Its aqueous solution is not precipitated by acetic acid, nor immediately by phosphoric acid (H3PO4). The solutions of many salts produce precipitates, among which may be named potassium cyanide (yellow), potassium ferrocyanide (dirty green), potassium chromate (yellow), and potassium iodide (yellow). It is not precipitated by either magnesium sulphate, copper sulphate, or ammonium oxalate.
Solution of picric acid and the soluble picrates precipitate the berberine completely from solution of hydrochlorate of berberine, with production of the insoluble picrate of berberine.
MONO-BERBERINE SULPHATE—BISULPHATE OF BERBERINE C20H17NO4.H2SO4.—This substance was introduced into medicine in America under the name Sulphate of Hydrastine. There are two sulphates of berberine (see di-berberine sulphate and phosphate of berberine), but as this is the one that has always been used under the name, it is the only sulphate recognized in commerce. Its medical value seems to be exactly that of muriate of berberine, but owing to its more soluble nature, it has nearly displaced that salt from market. Sulphate of berberine has been a favorite with physicians ever since its introduction.
Owing to the fact that this substance can be purified without the application of heat, and that it readily forms a soluble di-berberine sulphate by the action of dilute alkalies, from which other salts are easily prepared, we prefer to make this sulphate, and from it produce the various combinations.
Preparation.—Moisten any convenient amount of powdered hydrastis with officinal alcohol, and pack the powder properly in a suitable percolator which has previously been prepared for percolation. Exhaust the powder with alcohol, conducting the operation until the percolate does not contain enough berberine to repay the expense of manipulation and the loss of alcohol. [There can be no regular rule given for operations of this kind. The fineness of powder, packing of percolator, and temperature, influence the process to a considerable extent.] Reduce the temperature of the percolate to 50° F. (10° C.), and then gradually stir into it a decided excess of sulphuric acid. The natural combination of the alkaloid will be overcome, and a magma of fine crystals of berberine sulphate will immediately result. Permit the vessel to remain in a cool location for twenty-four hours, and then collect the sulphate of berberine on a filter or strainer of muslin. [When a concentrated aqueous extract of hydrastis in large amount is precipitated with excess of sulphuric acid, a finely divided, grainy precipitate of mono-berberine sulphate results. After this has subsided, white, needle-like crystals shoot out from the sides of the vessel and from the surface of the precipitate, which, when collected, washed with water, and dried, present a satin-like appearance. These are sulphate of calcium, and an examination of the precipitated sulphate of berberine, will show it to be largely contaminated with this substance which is also thrown down with it.] Reserve the filtrate for the preparation of the white alkaloid.
The sulphate of berberine at this stage of the operation is impure, being contaminated with free sulphuric acid, sulphate of calcium, a greenish oil which exists to a considerable extent in hydrastis, and with some other foreign substances.
Wash the sulphuric acid from the precipitate by means of cold alcohol; dry the precipitate, and mix it with sixteen times its weight of water; add ammonia water until in excess; allow the solution to stand a few hours, and then filter it. Add to the filtrate a slight excess of sulphuric acid, and collect the precipitated mono-berberine sulphate on a filter. Repeat this operation twice, and then dissolve the salt in boiling alcohol and crystallize it.
Description of Mono-Berberine Sulphate.—Excepting chromate of berberine, this sulphate of berberine is the darkest salt of the alkaloid known to us. When crystallized from a quickly cooled solution in boiling alcohol it forms beautiful groups of acicular crystals which, from their small size, have an orange yellow color. If the solution is less concentrated, and crystallization is conducted more slowly, the crystals are larger and of a deep orange color. When the crystals are of considerable size, an inch in length and an eighth of an inch in diameter, the natural color of the salt is that of a ruby, deeper than bichromate of potassium.
From what has been said it will be seen that the size of the crystals will alter the appearance of the salt. This may partly account for the discrepancy which exists in the writings of our authorities. In addition, the application of heat, as we have stated, will change the color, such action being undoubtedly accompanied by decomposition products. If an alcoholic solution be slowly cooled, crystallization commences with the formation of needle-like crystals, which, under certain conditions, will retain their characteristics until they cease to form. Under other circumstances, however, fan-like plates, resembling wasp wings, shoot out in most beautiful clumps, and, finally, perhaps another class of crystals will appear, consisting of granular nodules. These various forms are all sulphate of berberine, modified in appearance by different conditions of the solution and they are not different alkaloidal salts, a supposition once entertained by the writer. Upon separating them from each other, and severally dissolving them, all the modifications may crystallize from each solution, or the needle-like crystals may grow into the fan shape.
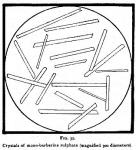 Figure 35 represents the microcrystals of mono-berberine sulphate, prepared by us, and drawn for this publication by Mr. W. J. Huck, under the supervision of Prof. F. B. Power, of the Wisconsin University. They were mounted in glycerine, and were in the form of distinct acicular prisms.
Figure 35 represents the microcrystals of mono-berberine sulphate, prepared by us, and drawn for this publication by Mr. W. J. Huck, under the supervision of Prof. F. B. Power, of the Wisconsin University. They were mounted in glycerine, and were in the form of distinct acicular prisms.
Mono-berberine sulphate is odorless, and imparts to the taste a pure, persistent bitterness, which is devoid of the nauseating properties of such substances as quassia or aloes. When the fine orange-yellow crystals are gently heated, they darken and change to a deep orange, but resume their original hue when the salt is cooled. It dissolves freely in ammonia water, and from this solution the mineral and some other acids in excess throw down precipitates of a salt of berberine and the acid employed. We take advantage of this fact in making other salts of berberine, as before remarked, for it is usually easier and more economical to prepare this sulphate and decompose it, than to prepare the other salts direct from the percolate.
Mono-berberine sulphate crystallizes from both water and alcohol in an anhydrous form. An exposure of eight hours to a temperature of 106° C. does not result in loss of weight. A higher temperature fuses and then decomposes it, a carbonaceous mass remaining.
Action of Reagents on Mono-Berberine Sulphate.—Dilute sulphuric acid, when boiled with mono-sulphate of berberine, does not immediately produce a red liquid. Cold concentrated sulphuric acid dissolves it, forming at first a greenish yellow, then a brownish, and finally a dark, almost black, liquid. Hot sulphuric acid dissolves it immediately with the production of a black liquid.
Cold hydrochloric acid dissolves a small portion of mono-berberine sulphate, forming a greenish-yellow liquid, which does not change upon boiling. If hydrochloric acid, or nitric, is added to an aqueous solution of mono-berberine sulphate, a flocculent mass of crystals of hydrochlorate of berberine, or nitrate of berberine, results. These do not dissolve upon the addition of an excess of ammonia water.
Hot glacial acetic acid freely dissolves sulphate of berberine, forming an orange liquid from which, upon cooling, a mass of fine crystals separate. These are freely soluble in ammonia water, from which solution either hydrochloric acid or nitric acid produce precipitates; sulphuric acid, however, under like circumstances, forms a dark brown liquid.
Hydroxide of ammonium dissolves mono-berberine sulphate freely and immediately, sulphate of ammonium and the di-berberine sulphate resulting. If to such a solution an excess of the ordinary acids be added, combinations of these acids and berberine results, with displacement of the sulphuric acid with crystallization of the berberine salt. Advantage may be taken of this fact to prepare other salts of berberine.
Carbonate of Potassium or Sodium solutions, if dilute, freely dissolve mono-berberine sulphate. Concentrated solutions of these substances decompose it, as with hydrochlorate of berberine.
In other respects, the remarks which we have applied to hydrochlorate of berberine may be applied to this sulphate.
Solubilities.—Mono-berberine sulphate dissolves slowly in water, After agitating one part of the salt for four days with seven parts of water, it was found that 100 parts of the solution contained 1.33 parts of the salt. If a mixture of mono-berberine sulphate and water be heated, however, it rapidly dissolves, forming a supersaturated liquid, which has a dark red color, and stains glass a deep orange. It can be filtered, and sometimes permitted to remain for some days in a cool situation, without other change than the deposition of aggregations of small grainy crystals. If a little sulphuric acid be added to this supersaturated liquid (sometimes only in minute amount to a portion of it), it immediately precipitates a magma of minute crystals of mono-berberine sulphate throughout the entire liquid, and the color of the supernatant liquid changes from red to yellow.
If mono-berberine sulphate be added to a ten per cent. solution of sulphuric acid in water, until an excess of one-eighth of undissolved sulphate is present, and the mixture be heated, the sulphate will entirely dissolve, and if the solution is permitted to cool, it will form a fine magma of minute crystals. If one part of mono-berberine sulphate be quickly dissolved in twenty parts of a hot five per cent. mixture of sulphuric acid and water, and then permitted to slowly cool, beautiful tufts of needle-like crystals result. If such a solution be digested for some hours at a temperature of 80° C, its color changes to brownish red, and upon cooling, only a small portion of the salt crystallizes. These crystals have a brown color.
Cold alcohol dissolves but a small amount of mono-berberine sulphate. After agitating one part of the salt for four days with seven parts of alcohol, the solution contained but 22 hundredths of one per cent. of the salt. Boiling alcohol, however, dissolves the salt rapidly, and in large amount, the solution being of a dark yellowish red in bulk, and orange-colored in thin layer.
Mono-berberine sulphate is insoluble in carbon disulphide, benzol, chloroform, concentrated ether, and is but slightly soluble in officinal ether.
Incompatibles.—Mono-berberine sulphate is incompatible with the mineral acids, tannic acid, gallic acid, salicylic acid, picric acid, and the soluble salts of these acids, forming precipitates when mixed with solutions of them. It is also incompatible with alkalies and the alkaline carbonates, being decomposed by these substances. Vegetable astringents usually produce precipitates with it.
DI-BERBERINE SULPHATE (NORMAL SULPHATE OF BERBERINE) (C20H17NO4)2 H2SO4.—This is the most beautiful salt of berberine. It has been used in medicine for some years, but never for a sulphate. A reference to the history of the alkaloid (p. 102) will show that its discoverer was probably Prof. Procter, although previous investigators were acquainted with the soluble compound that resulted when mono-berberine sulphate was added to diluted alkaline solutions. In our paper on phosphate of berberine, we introduce testimony which demonstrates that the substance sold in commerce under the name phosphate of berberine was in reality this compound. The so-called berberine (see p. 104) made according to the ammonia process was also the di-berberine sulphate. And we can not do better than to reproduce a portion of a paper contributed by us to the American Druggist [American Druggist, Wm. Wood & Co., Sept., 1884, p. 166.] on the subject:
"In 1878, a paper on the salts of berberine, as produced from Hydrastis canadensis, was presented to the American Pharmaceutical Association. [This was by the writer (J. U. Lloyd).] The writer announced that, when mono-berberine sulphate is added to ammonia water, by double decomposition a dark solution of berberine results, which, by mixing with alcohol, is mostly purified from the sulphate of ammonium which precipitates.... It is unnecessary to go over the properties of this substance in the present paper, as my object is to call attention to the fact that the substance obtained is not berberine, but a soluble sulphate of berberine. The writer has been aware of this fact for some years, but out of deference to an investigator who intended to consider the subject, waited for his report. However, this gentleman having withdrawn from the field, I feel at liberty to make the foregoing statement, and in addition announce the following:... There are two sulphates of berberine.... The existence of these two sulphates was announced in New Remedies, 1877, p. 226, by Mr. H. B. Parsons and Mr. T. J. Wrampelmeier."
Thus it is shown that the so-called berberine of our process of 1878 was in reality a di-berberine sulphate, and the equation expressing its formation is as follows: 2C20H17NO4.H2SO4 + 2NH4OH = (NH4)2SO4 + (C20H17NO4),2H2SO4 + 2H2O. Therefore, we introduce the process we presented at that time as follows:
Preparation of Di-berberine Sulphate. [In the original paper this is regarded as a process for making berberine.]—Rub eight parts of mono-berberine sulphate in a wedgewood mortar, cautiously adding ammonia water until in slight excess. Pour the dark liquid into thirty-two parts of boiling alcohol, and allow the mixture to stand thirty minutes, then filter. Stir into the filtrate thirty-two parts of cold concentrated sulphuric ether, and cover tightly. Surround the vessel with ice, and allow it to stand from twelve to twenty-four hours, then separate the magma of minute crystals of di-berberine sulphate with a muslin strainer or filtering paper, and dry by exposure to the atmosphere. Purify by crystallization from boiling alcohol.
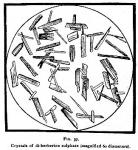
 Properties.—Di-berberine sulphate is an odorless, purely bitter, lemon-yellow, crystalline powder, or orange-colored crystals (it should not be red under these conditions). It crystallizes from boiling alcohol in beautiful clumps of yellow spangles, and is the finest salt of berberine known to us. (See figure 36.) When slowly crystallized in large crystals from a concentrated aqueous solution, it is garnet red. Figure 37 (next page) represents the micro-drawing made for this publication by Mr. W. J. Huck, under the direction of Prof. F. B. Power, who describes them as follows: "These crystals are somewhat larger than those of the mono-berberine sulphate, and of an entirely different shape, several of the crystals frequently coalescing. As represented in the drawing (fig. 37), the crystals are magnified 60 diameters, and were mounted in Canada balsam."
Properties.—Di-berberine sulphate is an odorless, purely bitter, lemon-yellow, crystalline powder, or orange-colored crystals (it should not be red under these conditions). It crystallizes from boiling alcohol in beautiful clumps of yellow spangles, and is the finest salt of berberine known to us. (See figure 36.) When slowly crystallized in large crystals from a concentrated aqueous solution, it is garnet red. Figure 37 (next page) represents the micro-drawing made for this publication by Mr. W. J. Huck, under the direction of Prof. F. B. Power, who describes them as follows: "These crystals are somewhat larger than those of the mono-berberine sulphate, and of an entirely different shape, several of the crystals frequently coalescing. As represented in the drawing (fig. 37), the crystals are magnified 60 diameters, and were mounted in Canada balsam."
 Di-berberine sulphate is soluble in ten parts of water from an excess of one-eighth part of the salt, and under the same conditions in 293 parts of alcohol. When slowly added to anhydrous alcohol it dissolves, and finally a yellow magma separates, which, after being dried, is very much less soluble in water than the di-berberine sulphate, and almost insoluble in anhydrous alcohol. The chemistry of this change has not been studied, but it is not a decomposition of the di-berberine sulphate, with the formation of a molecule each of berberine and mono-berberine sulphate, as might be possible, for, (C20H17NO4)2.H2SO4 = C20H17NO4 + C20H17NO4.H2SO4. In one instance a specimen of several ounces of crystallized di-berberine sulphate, that had been kept in a securely sealed vial for three years, became altered in properties, and almost insoluble in water.
Di-berberine sulphate is soluble in ten parts of water from an excess of one-eighth part of the salt, and under the same conditions in 293 parts of alcohol. When slowly added to anhydrous alcohol it dissolves, and finally a yellow magma separates, which, after being dried, is very much less soluble in water than the di-berberine sulphate, and almost insoluble in anhydrous alcohol. The chemistry of this change has not been studied, but it is not a decomposition of the di-berberine sulphate, with the formation of a molecule each of berberine and mono-berberine sulphate, as might be possible, for, (C20H17NO4)2.H2SO4 = C20H17NO4 + C20H17NO4.H2SO4. In one instance a specimen of several ounces of crystallized di-berberine sulphate, that had been kept in a securely sealed vial for three years, became altered in properties, and almost insoluble in water.
PHOSPHATE OF BERBERINE (C20H17NO4.7H4PO4+4H2O).—A substance was introduced into commerce by Dr. T. L. A. Greve, about the year 1877, under this name, and supposed to be a phosphate of berberine. It was in order to meet the demand for a more soluble salt than either the muriate or mono-berberine sulphate, which at that time were the only salts of berberine used in American medicine. This substance was made by Dr. Greve [Eclectic Medical Journal, Cincinnati, 1877, p. 311.] by digesting in boiling water a mixture of mono-sulphate of berberine, and precipitated phosphate of calcium, filtering, evaporating the filtrate to dryness, dissolving the residue in boiling alcohol to free it from sulphate of calcium, and evaporating the filtered alcoholic solution to dryness. [Dr. Greve states that, "The above process may also be varied by substituting phosphate of lead, or phosphate of barium, for the lime salt."]
In 1877, Prof. H. B. Parsons presented to the Michigan Pharmaceutical Association a process for making phosphate of berberine, and in connection with Mr. T. J. Wrampelmeier, followed it in New Remedies, 1878, p. 226, by an interesting paper on the subject.
They prepared a salt in accordance with the process of Dr. Greve, but found from an analysis that it was free from phosphoric acid, the "faint trace" present existing as an impurity in the form of bone ash. Subsequent analyses demonstrated that the salt was a sulphate of the composition (C20H17NO4)2.H2SO4. [Dr. Greve, therefore, struck upon the soluble di-berberine sulphate, which the writer also obtained, about the same time, by another process. Neither of us assigned it to its proper position.]
Messrs. Parsons and Wrampelmeier then made the soluble calcium orthophosphate. An excess of this acid calcium phosphate, CaH4(PO4)2, was then treated with mono-sulphate of berberine, when a precipitate of calcium sulphate resulted. The mixture was then evaporated nearly to dryness, and treated with hot diluted alcohol, whereby the remainder of the calcium salts were precipitated. The hot alcoholic solution of a berberine salt was then separated, by filtration, from the insoluble calcium salts, evaporated nearly to dryness, and then mixed with cold alcohol. A canary-yellow precipitate resulted, which, upon examination, proved to be a phosphate of berberine.
Phosphate of berberine was then made by Mr. Wrampelmeier, the acid phosphate of barium, BaH4(PO4)2, being used instead of the ortho-calcium salt. This product agreed in every respect with that obtained by the other experiment.
Analysis and Properties.—Phosphate of berberine exhibited a strong affinity for water, a long exposure, at from 67° to 70° C, being required to free it from moisture. The mean of two experiments, after the salt ceased to lose weight by an exposure of 100° C, resulted as follows:
| .2803 gram lost 0.181 gram=6.45 per cent. |
| .3034 gram lost.0200 gram=6.59 per cent. |
| Average loss at 100° C, 6.52 per cent. |
After destroying the organic matter by means of sulphuric and nitric acids, the phosphoric acid was estimated according to Fresenius' method, as magnesium pyrophosphate.
| .3034 gram gave.2145 gram Mg2P2O7= 1894 of H3PO44. |
| .2803 gram gave.2000 gram Mg2P2O7= 1766 of H3PO4. |
The average being 62.67 % of the phosphate of berberine employed.
The berberine was estimated by means of platinic chloride. According to Perrins, the precipitate has the formula 2C20H17NO4.2HCl.PtCl4. Of which 18.22 % is platinum, and 61.899 % is berberine.
| Phosphate of Berberine. | Precipitate. | Berberine. | Berberine estimated from platinum in ash | Berberine. |
| .2517 gram gave | .1312 gram= | .0812. | .0815. | 32.37 per cent. |
| .1727 gram gave | .0900 gram= | .0557. | .0526. | 30.45 per cent. |
| .1224 gram gave | .0640 gram= | .0396. | .0390. | 31.86 per cent. |
The platinum in the precipitates was then estimated from the ash, and the berberine calculated, which was considered more reliable than the preceding process, as it excluded a source of error in the tared filter paper. The result is shown in the last two columns of the above table. The average of berberine from the platinum of the ash being 31.56 %.
SUMMARY OF THE ANALYSIS.
| Percentage Found. | Percentage Calculated. | |
| Water (H2O) | 6.52 | 6.58 per cent. |
| Phosphoric Acid (H3PO4) | 62.67 | 62.76 per cent. |
| Berberine (C20H17NO4) | 31.56 | 30.65 per cent. |
| 100.75 | 99.99 per cent. |
These results give C20H17NO4.7H3PO4 + 4H2O, as the formula for phosphate of berberine. ["This formula seems, at first sight, an improbable one; but any person who will take the pains to look up the formula for the phosphates of the other alkaloids, will be surprised at their lack of uniformity, and at the fact that alkaloids exhibit no particular quantivalence."—PARSONS and WRAMPELMEIER.] The following equation expresses the reactions: C20H17NO4.H2SO4 + 6[BaH4(PO4)2] = C20H17NO4.7H2PO4 + BaSO4 + 5BaHPO4.
Continued on next page.
Drugs and Medicines of North America, 1884-1887, was written by John Uri Lloyd and Curtis G. Lloyd.

