Continued from previous page.
Nitrate of Berberine - Citrate of Berberine - Picrate of Berberine - Detection and Estimation of Berberine - Hydrastine (the White Alkaloid) - Yield of Hydrastine and Berberine
This being the only analysis of a compound of phosphoric acid and berberine known to us, we deemed it desirable to add further information to this subject. Accordingly, we brought the matter to the attention of Prof. Virgil Coblentz, who agreed to make an ultimate analysis of the compound, and in this connection we call attention to the fact that the salt was made by him by the direct combination of phosphoric acid and crystallized berberine, instead of by double decomposition. We therefore introduce the following report: [Prof. F. B. Power is also estimating the composition of phosphate of berberine, using a salt made by us, and crystallized from alcohol. Unfortunately, his report is not ready, and we will, therefore, present it to our readers at a future day in the Addenda.]
Preparation.—(Contributed to this publication by Prof. Virgil Coblentz). An accurately weighed quantity of the pure alkaloid, prepared by Prof. J. U. Lloyd, was dissolved in a sufficient amount of absolute alcohol, and into this solution exactly two grams of phosphoric acid (H3PO4) was weighed, the strength of which had been previously ascertained, two grams containing 1.2421 grams of absolute H3PO4. Then an equal bulk of absolute ether was added, and after allowing sufficient time for complete separation the mixture was thrown on a filter paper and the precipitate thoroughly washed with a mixture of alcohol and ether. The filter and contents were then removed and boiled in an excess of alcohol to remove all traces of adhering free acid, cooled, and mixed with its bulk of ether. The precipitate that formed was again thrown on a new filter and washed with a mixture of alcohol and ether until it was found to be free from uncombined phosphoric acid.
Gravimetric Estimation.—An amount of the alkaloid berberine weighing 0.460 grams was dissolved in absolute alcohol and treated as we have described. The liquids and washings were mixed and distilled water added; the ether and alcohol then evaporated by a gentle heat. To this aqueous solution of the free acid, ammonia water in slight excess was added and subsequently test magnesium mixture, until after having been well stirred and permitted to stand, no further precipitate followed the addition of the reagent. Ammonia water equal to one-fourth the volume of the liquid was then added, the vessel covered and allowed to stand for twelve hours. The precipitate of ammonio-magnesium phosphate was then collected on a filter and washed with a solution consisting of one part of officinal ammonia water and three parts of water, until the washings no longer produced a turbidity in a solution of nitrate of silver acidulated with nitric acid. The precipitate was then dried at 100° C, and ignited in a weighed crucible to low redness. From the weight of the resulting magnesium pyrophosphate (Mg2P2O7), the amount of phosphoric acid contained in the solution was calculated, 100 parts of Mg2P2O7 corresponding to 88.39 parts of H3PO4. 0.3297 grams of magnesium pyrophosphate were obtained from the solution, which corresponds to 0.2915 grams of phosphoric acid. Therefore, if from the 1.2421 grams of anhydrous H3PO4 contained in two grams of the phosphoric acid used we deduct the 0.2915 grams that remained uncombined, we have 0.9506 grams in combination with the berberine.
If one molecule of berberine C20H17NO4 (335), combined with one molecule of H3PO4 (99), 0.460 grams of berberine would require 0.13595 grams of phosphoric acid. In reality nearly 0.9513 grams of acid are required theoretically to represent seven molecules of phosphoric acid, as (.9513, .13595); this number corresponds closely to that actually found, 0.9506.
Four estimations were made in accordance with the foregoing scheme, resulting as follows:
| No. 1 gave 0.9506 grams of phosphoric acid (H3PO4), from 0.460 grams of phosphate of berberine. |
| No. 2 gave 0.9509 grams of phosphoric acid (H3PO4), from 0.460 grams of phosphate of berberine. |
| No. 3 gave 0.9508 grams of phosphoric acid (H3PO4), from 0.460 grams of phosphate of berberine. |
| No. 4 gave 0.9509 grams of phosphoric acid (H3PO4), from 0.460 grams of phosphate of berberine. |
The average, 0.9508, is practically close enough to the theoretical amount, 0.9513, to show that one molecule of berberine phosphate must contain seven molecules of phosphoric acid, therefore making the formula C20H17NO4.7H3PO4.
Volumetric Estimation.—This method depends on the indirect process of neutralization. 0.280 grams of the berberine were dissolved in absolute alcohol and phosphate of berberine was made as detailed on p. 124. The filtered mixture of alcohol and ether, containing the uncombined phosphoric acid, was then mixed with distilled water, and the ether and alcohol evaporated by a gentle heat. Into the aqueous solution that remained a normal solution of hydroxide of sodium was allowed to flow until sufficient of the latter was employed to insure the formation of the neutral sodium salt Na3PO4. Solution of chloride of barium was then added to this strongly alkaline liquid until no further precipitate was produced. After some hours, the resulting Ba3(PO4)2 was collected on a filter and well washed with water, the filtrate and washings being collected in a beaker. This was colored with solution of litmus and a normal solution of sulphuric acid was allowed to flow into it from a burette until a permanent pink hue resulted. The number of C. c. of normal acid solution required, deducted from the number of C. c. of the alkaline solution, was accepted as giving the amount of the latter required for the exact neutralization of the phosphoric acid; one C. c. of the normal alkali corresponding to 0.327 grams of anhydrous phosphoric acid.
SUMMARY OF THIS EXPERIMENT.
| 1.2421 | grams of H3PO4 | were contained in the 2 grams of acid used. | ||
| 0.6638 | " | remained uncombined. | ||
| 0.5783 | " | combined with the 0.280 grams berberine. | ||
| 42.3 | C. c. of normal solution | NaOH | used to neutralize | the free acid. |
| 22.0 | " | H3SO4 | excess of NaOH. | |
| 20.3 C. c. amount required for exact neutralization of uncombined H3PO4.
Hence, 20.3 C. c. x.0327=0.6628 grams of H3PO4 uncombined. |
||||
Four more experiments were made with the following result:
Experiment.
| No. 1. | 0.280 | grams berberine C20H17NO4 yielded of H3PO4 | 0.5790 grams. |
| No. 2. | 0.280 | " | 0.5788 |
| No. 3. | 0.280 | " | 0.5787 |
| No. 4. | 0.280 | " | 0.5788 |
If one molecule C21H17NO4 (335) combines with one molecule of H3PO4 (99), then 0.280 gram alkaloid would require 0.827, but we find practically that 0.280 gram of the alkaloid combines with on an average 0.5788 gram of the acid. Then, as 0.5788 / .0827 equals about seven, hence if theoretically 0.5792 gram of the acid combine with.280 gram of the alkaloid, and practically the amount found is about 0.5788 of acid, the formula must then be C20H17NO4.7H3PO4.
Properties.—Phosphate of berberine is a canary yellow powder, odorless and bitter. It changes to olive green when heated above 70° C., and gives up its water of crystallization at 100° C. It absorbs water upon exposure, and changes to a darker yellow, but does not deliquesce. Crystallized from hot alcohol, it forms irregular prismatic crystals. (P. and W.)
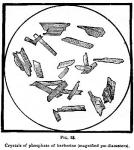 The crystalline structure of phosphate of berberine is represented by the micro-drawings (Fig. 38) made for our publication by Mr. W. J. Huck, under the direction of Prof. F. B. Power, who writes of them as follows: "The crystals are much broader than those of the preceding salts in consequence of the coalescence of several crystals, and the ends are very irregular in outline."
The crystalline structure of phosphate of berberine is represented by the micro-drawings (Fig. 38) made for our publication by Mr. W. J. Huck, under the direction of Prof. F. B. Power, who writes of them as follows: "The crystals are much broader than those of the preceding salts in consequence of the coalescence of several crystals, and the ends are very irregular in outline."
Solubilities.—One part of the crystallized salt dissolved in 10.43 parts of cold water.
One part of the salt dried at a temperature of 100° C. dissolved in 21.52 parts of cold water. It is almost insoluble in cold alcohol, and insoluble in pure alcohol, ether, and chloroform.
There is really no preference to be made for the pure phosphate over the di-berberine sulphate, which was really introduced for a phosphate. This fact has necessitated a longer paper than would be demanded by the phosphate in such a work as ours.
NITRATE OF BERBERINE. C20H17NO4.HNO3+H2O (Perrins).—Nitrate of berberine is sometimes used in medicine, but not as extensively as the muriate.
Preparation.—Nitrate of berberine is to be made according to the process employed in making the hydrochlorate, except that nitric acid is used instead of hydrochloric. This process can be followed even to the washing of the salt, for it is but slightly soluble in cold water. It should be dried, however, by exposure to a cool atmosphere, because decomposition follows when it is heated, especially if slightly moist.
Properties.—Nitrate of berberine obtained in this manner is in the form of a lemon yellow crystalline powder, odorless when fresh. It slowly dissolves in the mouth, imparting a bitterness to the taste. When kept for any length of time, especially during warm weather, or when heated in a test tube, it decomposes, evolving NO, and changes to a reddish color. The ultimate effect of this decomposition upon the constitution and properties of the residue has never been determined. According to Perrins it is perfectly stable at 100° C, but we have known it to decompose and evolve NO when kept in bulk at the ordinary temperature and become unfit for use. We do not consider it a desirable compound.
When nitrate of berberine is added to cold nitric acid, a dark brownish-red solution results, which, upon warming, changes to orange-red, with the evolution of an abundance of nitric oxide (NO). If one part of nitrate of berberine is gradually added to eight parts of hot nitric acid, nitric oxide is rapidly evolved, and finally the liquid changes from brown to a ruby red color. This solution has the characteristics which we have described (p. 113), under "Nitric Acid, Action on Hydrochlorate of Berberine," dissolving in the same solvents and contra, forming a yellow precipitate with water, and otherwise reacting so as to indicate that the products of decomposition in both cases are perhaps identical. Less heat, however, is required to produce the reaction between nitrate of berberine and nitric acid than between hydrochlorate of berberine and nitric acid.
Nitrate of berberine dissolves in cold sulphuric acid, forming a dark brown or black liquid, which does not change upon heating. Dilute sulphuric acid (1 to 7) forms a red liquid if boiled with nitrate of berberine. This color is not affected by an excess of hydrochloric acid, but is changed to brown by an excess of ammonia water. Nitrate of berberine dissolves slightly in cold hydrochloric acid. If a mixture of nitrate of berberine and hydrochloric acid be boiled, the salt is decomposed, a dark brown liquid resulting.
Hot glacial acetic acid freely dissolves nitrate of berberine with the production of a dark orange-colored liquid, which, upon cooling, deposits an abundance of yellow crystals. These dissolve freely in ammonia water, and from this solution hydrochloric acid precipitates masses of hydrochlorate of berberine. Under the same circumstances either sulphuric or nitric acid, with the aforenamed ammoniacal solution, forms a deep red liquid.
Nitrate of berberine will dissolve to an extent in cold ammonia water and more freely upon boiling, and crystallizes from the latter solution upon cooling. It corresponds with hydrochlorate of berberine in its deportment towards dilute or concentrated solutions of the hydroxides of potassium or sodium.
Nitrate of berberine is insoluble in benzol, carbon disulphide and concentrated sulphuric ether. It is slightly soluble in alcohol, and more so in water.
The saturated solution of nitrate of berberine in cold water has a greenish yellow color. From this solution most of the berberine in the form of crystalline salts is deposited by nitric, sulphuric or hydrochloric acid, but not by the addition of acetic or phosphoric (H3PO4), acid. No precipitate follows when magnesium sulphate, ammonium oxalate, lead acetate or copper sulphate are added to the aqueous solution, but a precipitate follows with potassium ferrocyanide (greenish), potassium chromate and potassium bichromate (yellow), and potassium iodide (yellow and gelatinous). Picric acid, picrate of ammonium, and solutions of the soluble picrates precipitate berberine completely from the solution of nitrate of berberine, the result being picrate of berberine.
 CITRATE OF BERBERINE—Preparation.—This may be made by direct combination between solution of citric acid and berberine. When a solution of one part of di-berberine sulphate is dissolved in sixteen parts of water and two parts of citric acid are added, and the solution permitted to stand for some days in a cool location, beautiful tufts of crystals are formed. These are of a fibrous, silky texture, very bitter, permanent, and are free from sulphuric acid. (Figure 39), next page. They have never been analyzed. Citrate of berberine is not very soluble in cold alcohol or water, but more freely in boiling.
CITRATE OF BERBERINE—Preparation.—This may be made by direct combination between solution of citric acid and berberine. When a solution of one part of di-berberine sulphate is dissolved in sixteen parts of water and two parts of citric acid are added, and the solution permitted to stand for some days in a cool location, beautiful tufts of crystals are formed. These are of a fibrous, silky texture, very bitter, permanent, and are free from sulphuric acid. (Figure 39), next page. They have never been analyzed. Citrate of berberine is not very soluble in cold alcohol or water, but more freely in boiling.
A cold aqueous solution of citrate of berberine has a greenish yellow color. Either sulphuric, hydrochloric, or nitric acid produces precipitates when added to this liquid. Citrate of berberine is not precipitated from aqueous solution by solution of magnesium sulphate, ammonium oxalate or copper sulphate. Precipitates result, however, from the addition of potassium iodide (gelatinous), potassium ferrocyanide, potassium chromate, potassium bi-chromate and by acetate of lead. This last (acetate of lead), differs from the reaction with nitrate of berberine. Picric acid or picrate of ammonium precipitates the berberine completely.
Citrate of berberine corresponds with nitrate of berberine and sulphate of berberine in its deportment towards concentrated sulphuric acid. If boiled with an excess of dilute sulphuric acid (1 to 7), the solution acquires a slight brownish tint and does not change by the addition of either hydrochloric acid or ammonia water.
Hydrochloric acid dissolves citrate of berberine to a slight extent, forming a yellow liquid, which is not changed by boiling.
Citrate of berberine corresponds with nitrate of berberine in its deportment towards glacial acetic acid and nitric acid.
Solubilities.—Citrate of berberine is insoluble in benzol, carbon disulphide, concentrated ether, and chloroform. It is slightly soluble in officinal ether.
Ammonia water is a good solvent for citrate of berberine, a reddish-brown liquid resulting.
PICRATE OF BERBERINE.—This substance is formed when picric acid, or a soluble picrate, is added to the solution of any other salt of berberine. This compound is not used in medicine outside of the Homoeopathic school, but it is of considerable interest to us as a test for berberine. We shall refer more fully to the characteristics of picrate of berberine hereafter.
Boiling distilled water dissolves very small portions of picrate of berberine, and upon cooling the solution, the salt separates entirely in crystalline form.
Picrate of berberine is insoluble in cold water, alcohol, ether, chloroform, benzol or carbon disulphide.
Nitric acid reacts with picrate of berberine in a manner similar to the action of that acid and hydrochlorate of berberine. The liquid which results from the action of hot nitric acid on picrate of berberine differs from that produced by hydrochlorate of berberine, as follows: With sulphuric acid it forms a red solution, which becomes lighter colored and cloudy on standing. It mixes with water in all proportions, forming clear solutions.
Sulphuric acid, hydrochloric acid and glacial acetic acid react with picrate of berberine similar to the manner in which they do with hydrochlorate of berberine.
Hydroxides of ammonium, sodium or potassium react as follows: Cold dilute solutions scarcely affect it, and boiling dilute solutions dissolve it very sparingly. Concentrated solutions of these alkalies dissolve it more freely, and if these solutions are rendered acid with sulphuric acid, a cloudiness results, which is dissipated by the addition of ammonia water.
Picric acid and the soluble picrates completely separate berberine and berberine salts from aqueous solution.
DETECTION AND ESTIMATION OF BERBERINE.—In the first natural order of plants, we have yet to consider two that contain berberine. These are Coptis trifolia and Xanthorrhiza apiifolia, and we shall introduce the processes for estimating the alkaloid when we reach the latter plant.
HYDRASTINE (THE WHITE ALKALOID OF HYDRASTIS CANADENSIS). C22H23NO6—History of Hydrastine.—In April, 1851, Mr. Alfred B. Durand published an essay in the American Journal of Pharmacy on Hydrastis canadensis. He had obtained, among other substances, a crystallizable body, and was inclined to view it as an alkaloid. With the light now before us, we know that his supposition was true, but in view of the fact that he could not make a crystallizable salt, he left the matter open, as follows: "For the present I shall therefore call the substance Hydrastine, with the hope that I will be more successful, after repeating my experiments on a large scale, in fully establishing its rank among the alkaloids." Since the alkaloid has not, as yet, yielded crystallizable salts with the acids Mr. Durand combined with it, viz.: nitric, hydrochloric, acetic and oxalic, it is not strange that he failed to obtain crystals. It seems that he neglected to continue his work; at least, he published nothing further on the subject. Hence, while the honor of the discovery belongs to Mr. Durand, the investigation of the character of the alkaloid and its salts must be placed to the credit of subsequent investigators; and in view of the opinions held by some persons, who believe that other parties discovered the alkaloid, we introduce a condensation of Mr. Durand's original process:
The crushed root of Hydrastis canadensis was macerated with cold water and then percolated with that menstruum, the percolate afterward being evaporated to dryness. 500 grains of the residue was dissolved in eight ounces of water, 125 grains of oxide of magnesium added, and the mixture digested on a sand bath for two hours, and then filtered. The residue within the filter paper was dried, digested in boiling alcohol, filtered, and the filtrate allowed to evaporate spontaneously. The result was, to use Mr. Durand's words, "beautiful, brilliant, yellow, four-sided, prismatic crystals, terminated by pyramidal summits."
In reviewing the process of Mr. Durand, it will be seen that, when the aqueous liquid obtained from the root was digested with magnesia, the acid then in natural combination with the white alkaloid united with the magnesia. This reaction was followed by precipitation of that alkaloid, which is insoluble in water, thus producing the "residue." This residue, upon being dried, was exhausted with boiling alcohol, in which menstruum, the alkaloid, is very soluble, and from it hydrastine was obtained in colored crystals by spontaneous evaporation of the alcohol. In describing these crystals, Mr. Durand identified the white alkaloid of hydrastis so clearly as to leave no doubt in the mind of any person familiar with the alkaloids of hydrastis. He states "it is insoluble in water, sparingly so in cold ether and alcohol, more so in ether when hot, entirely dissolved by chloroform and boiling alcohol."
The white alkaloid, hydrastine, is the only product of hydrastis that will conform to the foregoing description.
The color of Mr. Durand's alkaloid, a "brilliant yellow," was due to the presence of berberine, for that substance is most tenaciously held by hydrastine, and many re-crystallizations are necessary before it can be obtained colorless. (See preparation of hydrastine, p. 132, and Hale's "third alkaloid," p. 140).
Nothing was then written on the subject of hydrastine for a period of eleven years, although Prof. E. S. Wayne, of Cincinnati, made and presented to Prof. Procter (1856) a sample that, to use the words of Prof. Procter in the American Journal of Pharmacy, July, 1862, was "Identical in appearance and character with Durand's, except that it was lighter in color."
The next paper appeared in the American Journal of Pharmacy, July, 1862. The author, Mr. Wm. S. Merrell, speaks of having recently discovered two alkaloids in the rhizome of Hydrastis canadensis, and he proposed for them the names hydrastia and hydrastina. Both of these alkaloids had been discovered previously, one (hydrastia) being the well-known berberine. His description of that for which he proposed the name hydrastina, identified it as the alkaloid discovered in 1850, [Mr. Durand's paper was written in the summer of 1850, and published in 1851.] by Durand, and prepared in 1856 by Wayne. Mr. Merrell's proposed name could not, therefore, be accepted, and as the sample Mr. Merrell submitted to the editor of the Journal of Pharmacy was darker in color than either that made by Mr. Durand or Prof. Wayne, nothing was added to the literature on this subject.
The alkaloid had not yet been purified, all the parties reporting that it was either yellow (Durand and Wayne) or greenish (Merrell). The production of the pure alkaloid was reserved for Mr. J. Dyson Perrins, who announced it in the London Pharmaceutical Journal, May, 1862. He purified it by repeated crystallizations from hot alcohol, and described it as crystallizing in "four-sided prisms, and of great brilliancy," and he said of it, "the crystals are white."
Mr. F. Mahla, of Chicago, next contributed to the American Journal of Science and Arts, July, 1863, a paper on this alkaloid, and in 1878, the writer (J. U. Lloyd), read a paper before the American Pharmaceutical Association, on its preparation.
This brings us to the present year, and to the most important paper that has been written on the subject. It was by Prof. Frederick B. Power, of the University of Wisconsin, and was contributed to the American Pharmaceutical Association, 1884, and we shall make many references to this admirable treatise.
Preparation.—Hydrastine has always been made by decomposing the natural salt by means of an alkali. We introduce the process contributed by us to Prof. Power, by which we prepared the alkaloid examined by that gentleman, it is as follows:
"One thousand pounds of powdered Hydrastis canadensis were properly moistened with alcohol, packed in a suitable percolator, and percolation then conducted with the use of officinal alcohol as a menstruum. Sulphuric acid, in strong excess, was added to the percolate, and, after four hours, the supernatant liquid was filtered from the mass of crystals of sulphate of berberine (C20H17NO4. H2SO4). To this filtrate ammonia water was added until it showed but a slightly acid reaction, then strained to separate the precipitated sulphate of ammonium, distilled to a syrupy consistence, and the residue poured into ten times its bulk of cold water. After twenty-four hours the precipitated resinous substances, oils, etc., were separated from the liquid by filtration, the filtrate being an impure solution of sulphate of hydrastine. Ammonia water, in decided excess, was then added to this resultant liquid, and the precipitate of impure hydrastine collected and dried. It was then digested with one hundred times its weight of cold water, to which sulphuric acid was carefully added to slight acid reaction, and, after twenty-four hours, filtered. The filtrate was again precipitated with excess of ammonia water, the precipitate collected on a strainer and dried. This precipitate was powdered and extracted with boiling alcohol, from which impure, dark yellow crystals of hydrastine separated when the alcoholic solution was cooled. The crystals were purified by repeated crystallizations from boiling alcohol. In order to obtain the hydrastine perfectly colorless, when in the form of large crystals, many crystallizations are necessary."
Where the operator labors under the disadvantages of imperfect apparatus, thus entailing a great loss of alcohol, water can be used as a menstruum. However, under these circumstances, impurities are introduced that are not present when alcohol is used. The mother liquor from berberine sulphate (see page 117) can be used and adapted to this process.
 Crystalline Form and Appearances.—Hydrastine always forms imperfect crystals. Even when slowly crystallized, in large quantity, they are irregularly developed, and as they almost invariably form in such a way as to present their lateral surfaces to the solution, it is difficult to obtain good specimens. Figure 40 illustrates a few crystals that were selected from a batch of eight pounds of the alkaloid, and present the most perfect of the specimens, and as
Crystalline Form and Appearances.—Hydrastine always forms imperfect crystals. Even when slowly crystallized, in large quantity, they are irregularly developed, and as they almost invariably form in such a way as to present their lateral surfaces to the solution, it is difficult to obtain good specimens. Figure 40 illustrates a few crystals that were selected from a batch of eight pounds of the alkaloid, and present the most perfect of the specimens, and as 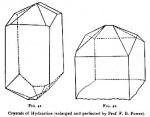 they appeared in the solution. They are such as Prof. Power employed in making his measurements. The following description is that of Prof. Power, and figures 41 and 42 represent the perfected crystals as constructed by that gentleman.
they appeared in the solution. They are such as Prof. Power employed in making his measurements. The following description is that of Prof. Power, and figures 41 and 42 represent the perfected crystals as constructed by that gentleman.
"The crystals, which attain a maximum length of from eight to ten millimeters, have the form of four-sided prisms (Fig. 41 and 42), and apparently belong to the ortho-rhombic system, although the goniometer at my disposal did not admit of the exact measurement of the angles. The drawings here presented, which represent typical crystals, were formed by making an orthographic projection, and the angles may be said to be as geometrically accurate as is possible to obtain them without absolute measurements. In Fig. 42 the terminal faces are shown to be very perfectly developed, while Fig. 41 represents a crystal as viewed somewhat from the side and from above, the terminal faces not so symmetrically developed, and therefore having a somewhat more complicated form. It is interesting to observe that when both ends of the crystals are developed, as shown in Fig. 41, the corresponding terminal faces of opposite ends are invariably inclined to each other at an angle of exactly 90°."
Hydrastine can be crystallized in glassy crystals, perfectly colorless and very brilliant. As a rule, however, the crystals are opaque and white, owing to the presence of numerous fractures. When in this form and in small crystals, it may be quite colored and appear white.
Chemistry of Hydrastine.—The first analysis was made by Mr. F. Mahla, [Silliman's American Journal, Vol. 36, No. CVI., p. 57.] who assigned to it the composition C22H24NO6. From his figures Kraut deduced the formula C22H23NO6 [See Power's paper on Hydrastine, Proceedings of the American Pharmaceutical Association, 1884.]. Thus it is that the investigation of Prof. Power, in 1884, is the second published contribution to the subject, although Prof. J. F. Eykman, of Tokio, Japan, has investigated the subject, but has not, as yet, published the results of his analysis. [We are informed by Prof. Eykman that he is especially interested in the decomposition products of this alkaloid, and we hope to one day to present his paper.] Prof. Power's analysis coincided very nearly with that of Mr. Mahla, although he followed a different method for making his determination. The following table compares the results of the analyses:
| Calculated for C22H23NO6 | Prof. Power found. | Mr. Mahla found. | |
| C=66.48 per cent. | 66.69 per cent. | 66.69 | 66.38 |
| H= 5.79 per cent. | 5.61 per cent. | 6.01 | 5.69 |
| N= 3.53 per cent. | 3.46 per cent. | 3.83 | 3.76 |
| O=24.20 per cent. | - | - | |
| 100. | |||
Prof. Power states that "The results of both our analyses are seen to agree quite closely with the accepted formula, which may, therefore, now be presumed to be correct."
Properties of Hydrastine.—Hydrastine unites with the acids, and forms salts, none of which have as yet been crystallized. Prof. Power failed to produce a crystal, and we have exposed large amounts of the muriate, sulphate and citrate, to the most favorable conditions, and to a temperature of -28° C. in lots of ten pounds, without success. By spontaneous evaporation a glassy substance invariably remains, destitute of crystalline form.
When a salt of hydrastine is dissolved in water and then precipitated by an alkali, the result is a bulky amorphous magma of the alkaloid. This begins to shrink in bulk in a short time, and finally assumes a crystalline form, when the product will occupy but a small proportion of the bulk of the original magma. The addition of alcohol to such a precipitate hastens the change from the amorphous to the crystalline. Impure hydrastine precipitates white, owing to the minute division, but becomes very dark after assuming the crystalline form, carrying the coloring matters with it. For this reason it is not practical to purify the alkaloid by repeated solutions in acid water and precipitations with an alkali. Although mono-berberine sulphate is quite soluble in dilute ammonia water (forming the di-berberine sulphate), and hydrastine is perfectly insoluble in that menstruum, it is impossible to separate the berberine from sulphate of hydrastine by the method of precipitation. The tenacity with which the hydrastine holds this yellow alkaloid under these conditions led the writer to doubt for a long time the identity of this yellow substance and berberine (and others have been misled); but by repeated crystallizations of impure (yellow) hydrastine from boiling alcohol, a deep yellow liquid was obtained that, upon purification, yielded a considerable amount of berberine. One experiment, wherein a batch of six and one-half pounds of impure hydrastine was worked, yielded three and one-half ounces of mono-sulphate of berberine. (See Hale's "Third Alkaloid," p. 142.)
Hydrastine is tasteless if the saliva is of alkaline reaction. Its soluble salts are acrid.
Action of Reagents on Hydrastine.—According to Prof. Power, "The crystals of hydrastine are affected in the following manner by reagents:
"Concentrated sulphuric acid produces a yellow color, which, in contact with a crystal of potassium bichromate, becomes brown. Concentrated sulphuric acid, on warming, produces a bright red color. Concentrated nitric acid produces, in the cold, a yellow color, changing to reddish-yellow. Concentrated hydrochloric acid gives no coloration, either in the cold or upon warming. Concentrated sulphuric acid and monolybdate of ammonium gives an olive-green color, which appears to be its most characteristic test."
The solution of the hydrochlorate is affected as follows by reagents (Power):
"Ammonia water and the fixed alkalies give a white, curdy precipitate, sparingly soluble in excess; potassium iodide, potassio-mercurio iodide, potassium ferrocyanide, potassium sulphocyanide, mercuric chloride and tannic acid produce white precipitates; iodine and potassium iodide, a light brown precipitate; potassium bichromate, a yellow precipitate; picric acid, a bright yellow precipitate; platanic chloride, an orange yellow precipitate; auric chloride, a deep yellowish-red precipitate."
Decomposition Products of Hydrastine.—Crystals of hydrastine fuse "at 132° C. (Mahla states 135° C.), to a light amber-colored liquid. When heated on platinum-foil they decompose with the evolution of empyreumatic, inflammable vapors, reminding, as Mahla had previously observed, somewhat of carbolic acid, and leaving a large amount of ash, which burns slowly away at a red heat. In order to ascertain whether hydrastine is capable of yielding a hydro compound, five grams of the alkaloid were dissolved in dilute sulphuric acid, and subjected for about two days to the action of nascent hydrogen, as developed from metallic zinc and platinum, The liquid was then filtered, precipitated by ammonia water, in slight excess, and the precipitate, after washing, dissolved in hot alcohol, and allowed to crystallize. The crystals are insoluble in water, and closely resemble in appearance those of hydrastine, but possess a slightly yellowish tint, which could not be removed by repeated crystallization. The melting point also lies close to that of hydrastine, being observed at 131° C. I have not as yet subjected these crystals to ultimate analysis, but have formed therefrom and analyzed the hydrochlorate. The latter, like the hydrochlorate of hydrastine, is amorphous, and remains, by the evaporation of its solution, in the form of a transparent, yellowish varnish, yielding, however, a nearly white powder, freely soluble in water. After drying at 100° C., 0.7830 gram of substance gave 0.2560 gram AgCl=0.0651 gram HCl., or 8.31 %.
"This result would therefore indicate that a hydro-hydrastine is thereby formed, by the absorption of four atoms of hydrogen, and is analogous in composition to hydroberberine, C20H21NO4 (Ann. Chem. Pharm. Suppl., 2, 191).
| Calculated for C22H17NO6HCl. | Found. |
| HCl=8.34 per cent. | 8.31 per cent." |
—Power.
Prof. Power also formed combinations of hydrastine and both bromine or iodine, such reactions being accompanied by the evolution of considerable heat, but he did not determine the composition of such compounds. By distilling a mixture of the alkaloid and caustic potash, unpleasant, inflammable vapors escaped, and a yellowish brown mass remained. Upon dissolving this in water and adding sulphuric acid until in slight excess, and distilling the liquid, formic acid was detected in the distillate. The residual acid liquid upon agitation with ether, and evaporation of the ethereal solution, yielded protocatechuic acid (C7H6O4); and no other acids were identified. (This acid is also obtained as a decomposition product of berberine; see p. 109).
Upon treating hydrastine in alcoholic solution with ethyl iodide and subjecting it to heat, hydriodic acid was evolved, and the reddish yellow syrup that remained upon dilution with alcohol deposited a white crystalline powder. This dissolved freely in warm water, and crystallized colorless upon cooling. These were anhydrous, fused at about 183° C., but underwent decomposition by the application of heat. An analysis of this substance demonstrated that it had the composition C22H22(C2H5), NO6HI, and was evidently the hydriodate of ethyl-hydrastine. Since this compound was formed by the substitution of the ethyl radical (C2H5), for one atom of hydrogen of the molecule of hydrastine, Prof. Power considers hydrastine to be a secondary or imide base, and he writes as follows:
"In this respect, according to Henry [Ann. Chem. Pharm. 115, p. 132.] and Bernheimer, [Gazz, Chim. Ital. xxiii., pp. 329-342.] it occupies an analogous position to berberine, since they obtained from the latter mono-ethyl and methyl derivatives, while, according to Perrins and Schmidt, in the case of berberine, the simple hydriodate of the base is thereby formed.
"That the crystalline compound obtained from hydrastine is really an ethyl derivate is evident, not only from the analysis, but I have also prepared the simple hydriodate by dissolving the alkaloid in freshly prepared hydriodic acid. As thus obtained, it is an amorphous substance, and very easily decomposed."
Solubility of Hydrastine.—Hydrastine is perfectly insoluble in water, or dilute alkaline solutions. Chloroform dissolves it freely, and is the best solvent we have found. It also dissolves in benzol, ether and cold alcohol, and freely in boiling alcohol. According to Prof. Power, it is insoluble in petroleum benzine, and its relative solubilities in the following liquids areas follows: One part of hydrastine in 1.75 parts of chloroform, in 15.70 parts of benzol, and in 120.27 parts of cold alcohol. Hydrastine unites with acids to form salts which are mostly soluble, tannic acid and picric acid forming insoluble combinations. These artificial salts are of acid reaction.
Salts of Hydrastine.—Muriate of hydrastine is used in medicine more extensively than any other salt, but the citrate is in some demand. These are both very soluble, and are colorless, although if a prolonged temperature be applied to the muriate, even if not above 82° C, it turns yellow. Alkalies decompose solutions of the salts of hydrastine, the alkaloid being precipitated.
These salts are best prepared by dissolving the acid in alcohol, and then adding an excess of hydrastine. After the solution ceases to take up the alkaloid, it is filtered and brought, if necessary, to a very slight acid reaction by means of the acid employed, and then evaporated at a low temperature to dryness. Salts of hydrastine and some of the volatile acids are not permanent, but decompose upon drying them, the acid escaping. Prof. Power calls attention to this fact with acetic acid. The composition of the salts of hydrastine are as follows: Muriate of hydrastine C22H23NO6.HCl (Mahla & Power). Double chloride of hydrastine and platinum (C22H23NO6.HCl)2+PtCl (Mahla & Power). Sulphate of hydrastine (C22H23NO6)2.H2SO4 (Power) Double chloride of hydrastine and gold (C22H23NO6.HCl)2AuCl3 (Power).
YIELD OF HYDRASTINE AND BERBERINE FROM HYDRASTIS CANADENSIS.—The proportion in which these substances exist in hydrastis is quite variable. The season of year in which the rhizome is gathered, the method of curing the drug, and its age, being instrumental in varying the amounts of the alkaloids. If the drug is gathered in July or August, and quickly dried in the shade, it is in the best and most valuable condition. If it is gathered in the spring of the year, it is of inferior quality. Under any circumstance, carelessness in curing of the drug injures it, and may render it completely worthless. We have a constant experience in this variability of quality, and every year are compelled to reject considerable amounts that will not, in yield of alkaloids, repay the expense of working the material (see p. 95). That such a drug is not lost to the world may be inferred by consulting the table that we offer under powdered hydrastis, and it is to be hoped that in a day to come hydrastis (and other American drugs) may command a price in accordance with its real value.
The amount of berberine that exists in hydrastis is also influenced by the length of time the rhizome has been exposed to the atmosphere. It is constantly decomposing, even though the drug is stored in a comparatively protected position (see pp. 84 and 85). There seems to be a kind of decay that finally will result in the destruction of a considerable amount of berberine. In order to determine the progress with which this decay continues, we selected, 1870, pounds of freshly gathered, dried, hydrastis. Of this lot 500 pounds were worked at once, in a single percolatior; 700 pounds in like manner were worked in twelve months, and 670 pounds in twenty-four months. Every precaution was taken to insure the same manipulation with each batch. The result was as follows:
| The | first batch, | 500 pounds, yielded | 9 pounds of mono-berberine sulphate, or | 1.8 per cent. |
| second | 700 | 9 3/4 | 1.39 | |
| third | 670 | 9 | 1.34 | |
| Average of | 1870 | being 27 3/4 | 1.48 | |
(The average yield of commercial hydrastis is from 18 to 28 ounces of sulphate of berberine to the hundred pounds. It is not profitable to carry the extraction to the utmost limit, and from 18 to 24 ounces is a fair product.)
Hence it follows that it is not economy to store hydrastis from year to year, and manufacturers of these alkaloids have learned to work the drug while recent. Regarding the white alkaloid, hydrastine, we can not present a similar line of comparison. It is our custom to reserve several batches in crude form, and work the product of about 5,000 pounds of hydrastis at once. The yield of purified hydrastine, perfectly white crystals, averages from one-fourth of one per cent. to three fourths of one per cent. of the drug employed.
In this connection we will remark, that we have often noticed that batches of the drug which gave unusually low amounts of berberine, were liable to yield an increased amount of hydrastine. Mr. J. W. Forbes informs us that he has also noted this peculiarity, in working the drug in large amounts. In one instance (recorded by us in 1879) a lot of 1,000 pounds of ground hydrastis was moistened with water, and, by an accident, only half of it could be worked at once. The remainder became heated and changed in appearance (becoming greener in color), and when it was worked, the berberine proved to have mostly perished. The result, however, was a yield of hydrastine very much in excess of that obtained from the other half of the drug.
These circumstances, taken together, would suggest that there was a natural connection between the alkaloids, the indication being that, if such is the case, hydrastine is produced in the economy of the plant, by the disintegration of berberine. Prof. F. B. Power, in reviewing the analyses of these alkaloids, is inclined to view this relationship as complex, if it exists at all, and he writes: "It is also quite evident that there is no simple relationship between hydrastine and the alkaloid berberine, C20H17NO4, such as exists, between the associate alkaloids, morphine and codeine, or caffeine and theobromine." It must be admitted that, if such changes occur, they are perfectly obscure and beyond the light of our present knowledge of the chemistry of these substances. It must also be recognized that there are several constituents in hydrastis that, together with their decomposition products, are unknown. In this connection we are sometimes led to compare together the plants that yield berberine, and it is usual to find the alkaloid associated with more or less of another alkaloid. It is not unreasonable to infer that a connection exists between them.
Continued on next page.
Drugs and Medicines of North America, 1884-1887, was written by John Uri Lloyd and Curtis G. Lloyd.

