 PARTS USED.—The dried flowering plant and the seeds of Lobelia inflata, Linnaeus.
PARTS USED.—The dried flowering plant and the seeds of Lobelia inflata, Linnaeus.
Natural Order Campanulaceae, Tribe Lobelieae.
![]() BOTANICAL DESCRIPTION.—Lobelia is an annual herb growing in dry fields and pasture grounds and woodland pastures. In dry sunny places it attains a height of a few inches to a foot or two, the usual height in pasture lands being about a foot. In shady, rich soil, however, it is more luxuriant, growing two or three feet and becoming more slender and fewer branched. The plant flowers in August continuing until frost into September. When the time to flower arrives, each plant begins to bloom, no matter what its height or size. Often plants will be found in bloom only an inch or two high, and only bearing three or four small leaves and as many terminal flowers. Our figure 126 represents such a plant.
BOTANICAL DESCRIPTION.—Lobelia is an annual herb growing in dry fields and pasture grounds and woodland pastures. In dry sunny places it attains a height of a few inches to a foot or two, the usual height in pasture lands being about a foot. In shady, rich soil, however, it is more luxuriant, growing two or three feet and becoming more slender and fewer branched. The plant flowers in August continuing until frost into September. When the time to flower arrives, each plant begins to bloom, no matter what its height or size. Often plants will be found in bloom only an inch or two high, and only bearing three or four small leaves and as many terminal flowers. Our figure 126 represents such a plant.
 The roots of Lobelia are few and fibrous. The stem is erect, green, round, striate and covered with sparse white hairs, that are beautiful objects under a microscope. Each stem that attains the usual size is branched about the middle with several ascending branches, axillary from the leaves, and ending each in a spike of flowers. The branches are always much shorter than the main stem.
The roots of Lobelia are few and fibrous. The stem is erect, green, round, striate and covered with sparse white hairs, that are beautiful objects under a microscope. Each stem that attains the usual size is branched about the middle with several ascending branches, axillary from the leaves, and ending each in a spike of flowers. The branches are always much shorter than the main stem.
The leaves are alternate, mostly sessile, or the lower short stalked, and slightly decurrent down the stem; they are obvate or oblong, usually an inch to two long and half as wide, varying smaller till they merge on the upper part of the stem into flower bracts; they are of a light green color, downy on both sides and soft to the touch. The veins are numerous, projecting below the leaf and impressed in the upper side of it. The margin is erosely blunt-toothed, the teeth tipped with small glandular white tips.
 The flowers appear in August, the first to open axillary to the upper leaves which become successively smaller, passing into the bracts of a terminal raceme. The flowers themselves are rather inconspicuous being only about a quarter of an inch long. They are bourne on short, erect peduncles about the length of the calyx lobes.
The flowers appear in August, the first to open axillary to the upper leaves which become successively smaller, passing into the bracts of a terminal raceme. The flowers themselves are rather inconspicuous being only about a quarter of an inch long. They are bourne on short, erect peduncles about the length of the calyx lobes.
The calyx is adherent, with a globular ribbed tube and five slender, linear, sub-equal, erect teeth, which are nearly as long as the corolla. The corolla is small, bilabiate, and of a light blue color; the tube of the corolla is split the entire length on the upper side, a characteristic of all the species of Lobelia, the upper lip consists of two erect, narrow lobes, the lower of three sub-equal, broad reflexed segments. The stamens are five and cohering together, both filament and anther, around the pistil, form a column the length of the corolla tube and slightly projecting from the split in this tube. The pistil consists of a two-celled, inferior ovary, containing numerous minute ovules attached to the large central spongy placentas, and completely filling the ovary when in flower. The style is enclosed in the tube formed by the stamens, and ends in a small two-lobed stigma.
![]()
 The fruit-pod is a peculiar shape, as shown in our figure 129. It is about a quarter of an inch long, inflated, sub-globular, compressed laterally, and unequal at the base, the cell opposite the stem being longer at the base than the inside cell. This is characteristic of the fruit. [Bentley & Trimen's illustration of the pod (fig. 6, plate 162, also of the pods on the stem) is inaccurate, as it represents the pod equal at the base, and large at the apex tapering to the base, (club shape,) which is not the case.] The pod is prominently ten veined lengthwise with numerous, intermediate, net veins. It is crowned with the five persistent linear calyx segments, which on the unripe pods are nearly erect and slightly more than half the length of the pod; the sides are very thin and easily compressed. The pod is very much inflated, (whence the name of the plant,) and is divided lengthwise into two cells by a thin partition; it contains an axial two-lobed, comparatively large, spongy placenta, which is densely covered with the numerous minute seeds. The description and illustration of the seeds are given in our description of the drug.
The fruit-pod is a peculiar shape, as shown in our figure 129. It is about a quarter of an inch long, inflated, sub-globular, compressed laterally, and unequal at the base, the cell opposite the stem being longer at the base than the inside cell. This is characteristic of the fruit. [Bentley & Trimen's illustration of the pod (fig. 6, plate 162, also of the pods on the stem) is inaccurate, as it represents the pod equal at the base, and large at the apex tapering to the base, (club shape,) which is not the case.] The pod is prominently ten veined lengthwise with numerous, intermediate, net veins. It is crowned with the five persistent linear calyx segments, which on the unripe pods are nearly erect and slightly more than half the length of the pod; the sides are very thin and easily compressed. The pod is very much inflated, (whence the name of the plant,) and is divided lengthwise into two cells by a thin partition; it contains an axial two-lobed, comparatively large, spongy placenta, which is densely covered with the numerous minute seeds. The description and illustration of the seeds are given in our description of the drug.
COMMON NAMES.—The drug is now known to the drug trade as Lobelia or Indian Tobacco.
A number of names have been applied to the plant, mostly in old works. The earliest botanists did not use a common name for it. Aiton, (1810,) calls it Bladder Pod, and this name with Inflated Lobelia and Bladder Pod Lobelia, are the natural translations of the specific name, hence, the ones used at first by botanists.
From its taste which resembles tobacco the plant began to be known as Wild Tobacco to the people, and this name was used in Eaton's first Manual of Botany, and carried through all the successive editions. From Wild Tobacco it is quite natural that it should acquire the name Indian Tobacco, as it would be presumed a tobacco that was wild would be used by the Indians. As a matter of fact, however, we have no record that the Indians ever made use of the plant in the manner of a tobacco. Dr. Carver, who spent a greater part of his life among Indian tribes, and, who wrote a list of the various economic plants used by them, does not mention the plant. Indian tobacco began to be applied about 1814, (Biglow,) but did not come into general use, outside of medicine, until adopted in the botanical class books; first, by Beck, 1833; then Wood, 1845, and Gray, 1848. At the present time it is the only common name applied to the plant, either in medicine or botany.
On the introduction of the plant to medicine a new series of common names, denoting its properties were originated.
Thomson and Cutler, who first brought the plant to general attention, called it Emetic weed, and from this name Puke weed, Vomit weed, and Gag root, have been suggested and used.
We find the name Asthma weed applied by a few writers, and in very old works, Eye-bright. In our article on the medical history will be found further remarks in connection with this subject.
BOTANICAL HISTORY.—GENERIC.—The genus Lobelia is a very large family of plants, distributed mostly in tropical and sub-tropical countries, and a few found in temperate and even frigid zones.
They are characterized by a uniformity in the structure of the flowers and fruit, but differ widely in general habits, which has given rise to a number of sections, considered distinct genera by various authors.
Plants of this genus have all milky juice, a five-lobed calyx, an irregular two-lipped corolla with the tube slit along the upper side, and five anthers united around the style. To a mere novice in botany, plants of this family can be recognized by the very peculiar split corolla and the united stamens.
The position of the genus in the natural system is obviously near the great family Compositae, and has so been placed in all systems of classification. The genus agrees with the family in the trifid style, the anthers united around the stigma and the adherent ovary; with the tribe Cichoraceae; in having milky juice and the corolla split, the segments cohering together in one piece; with the tribe Mutisiaceae in having labiate flowers: [It is a fact, not generally known to our botanists, because their attention is not directed to it by any common native plants, that a large section of the Compositae, consisting of over fifty genera of South American and African plants, are chiefly characterized by having bilabiate corollas. We have in our Southern States a single species (Chaptalia tomentosa. Vent.) that belongs to this section.] it differs m having the flowers not collected in an involucrate head, which at first makes them appear very different, and in the character of the ovary.
The genus Lobelia has always been considered a type of a natural order, Lobeliaceae, established by Jussieu, 1811, [Memoire sur les Lobeliacées et les Stylidiées, nouvelles families des plantes, A. L. de Jussieu, Annals des Sciences Naturales, Paris, vol. xviii, 1811.] and maintained by Endlicher, De Candolle, and most systematists, including all writers on American botany, even Dr Gray in his very recent work, 1878. [Synoptical Flora of North America, Asa Gray, New York, 1878, vol. ii., part i, page 2. Dr. Gray says on this subject "Too near the Campanulaceae and nearly passing into them, therefore united by recent authors; but as there are two dozen genera, agreeing in the indefinite inflorescence, irregular corolla and mostly in the syngenesious anthers, it seems best to retain the order".] By Bentham and Hooker, however, 1876, [Genera Plantarum, Bentham and Hooker, vol. ii., (part 2, 1876,) p. 551.] these plants are included as a tribe Lobelieae, of the natural order Campanulaceae and we have followed these authors to give uniformity to our work, theirs being the last general work on plant classification that has been published. [We will state in this connection that we think the family a perfectly natural one, and distinct from the Campanulaceae. Indeed, any one will have more trouble in finding points of resemblance than points of difference between the two sections.
While we would like to follow all American authority, the Pharmacopoeia, all our medical works and our own views in considering the family distinct still, we think it better to adopt the classification of Bentham and Hooker, acknowledging them as the present botanical authority on the classification of the plants of the world.]
In old times plants of this genus were described in common with widely different ones under the family name of Rapunculus. It was Tournefort, who first clearly defined the genus in 1719, [ Institutiones Rei Herbariae, J, P. Tournefort, Paris, 1719, p. 163, plate 51.] giving it the name Rapuntium and as this genus is very natural and most of the species are still retained, it is unfortunate that the name has been replaced. The history of the present name of Lobelia is as follows: In 1703 Charles Plumier [See note *, p. 21. Plumier was the first to honor living persons by introducing their name into scientific nomenclature. The plan met with much opposition at first because it was liable to be abused, and names of persons selected, who's scientific labors do not entitle them to this distinction. It has been adopted by many of the most eminent botanists.] in his work on plants of the West Indies, [Nova Plantarum Americanarum Genera, P. Carolo Plumier, Paris, 1703, p. 21 and plate 31.] dedicated to his friend Matthias de Lobel, * a genus founded on a plant collected in the West Indies. Linnaeus referred this plant to Tournefort's genus, Rapuntium, and adopted the name Lobelia for the genus, probably because it was the prior name Afterwards, when his attention was directed to the fact, that under the name Lobelia, a large number of plants were included entirely distinct from the original plant described by Plumier, Linnaeus deemed it best to retain the name for the plants to which it had become most generally known and to originate a new name for the genus of Plumier. [This genus is Scaevola, established by Linnaeus. and referred to the natural order Goodenovieae The genus has a cleft corolla tube, similar to Lobelia, which no doubt led Linnaeus to originally place them together, but the fruit is very different, being in Scaevola a fleshy drupe containing a single large seed. Plumier's plate shows quite plainly the nature of the fruit which would exclude his plant from the present genus Lobelia.
In thus transferring a generic name from the original species to which it was given, to a family to which it had become attached we find an analogous case in the name Magnolia. (See note, page 21.)]
BOTANICAL HISTORY.—SPECIFIC.—The original collector of Lobelia inflata is not known, but it was evidently sent to Europe early in the last century. The first authentic mention we can find of it is by Linnaeus (1737) [Lobelia caule erecto brachiato, foliis ovato-lanceolatis obsolete incisis, capsulis inflatis.—Linnaeus, Hortus Cliffortianus, 1737, page 500. It is not stated whether the plant was growing in Clifford's garden at that time, or whether it was merely preserved in his herbarium, as the Hortus Cliffortianus describes both plants of his garden and dried collection.] in his catalogue of the plants in the garden of George Clifford, ** hence, it was evidently in cultivation at that time. It is probable that Tournefort also refers to this plant, (1719,) [Rapuntium Americanum, Virgae aureae foliis, parvo flore subc eruleo."—Tournefort, Institutiones Rei Herbariae, Paris, 1719, p. 163.] but we can not say with certainty.
Previous to the appearance of Linnaeus' [Species Plantarum, 1st edition, 1753 page 931.] "Species Plantarum" (1753,) the plant was specified by a number of descriptive adjectives. [Lobelia caule erecto, foliis ovatis subserratis, pedunculo longioribus, capsulis inflata.—Linnaeus, Hortus Upsaliensis, 1748, p. 276.] Linnaeus named it Lobelia inflata from the inflated seed-pods which name it has retained to the present day with the single synonym of Rapuntium inflatum given to it by Miller, but used by no one else.
DESCRIPTION OF THE DRUG.—In commerce two products of the plant are found, the entire dried herb and the dried seed. The former only is officinal, but the seed is a distinct article of trade, and very largely used. [The powdered herb was known to Thomsonians as green lobelia. The powdered seed as brown lobelia.]
Lobelia Herb.—As found in commerce this drug consists of the stems, leaves, and inflated capsules of Lobelia inflata. Usually the plant is gathered after the lower leaves have changed to brown and often the seeds have fallen from the lower capsules, which are then also brown. The plant is cut off just above the ground and the lower portion of the stem is generally devoid of leaves even in the carefully selected recent drug. Sometimes the plant has been known to appear so abundantly over an old field as to permit of its being mown with a scythe, [Prof. A. J. Howe relates to us an instance in which several tons were cut at one time from an old wheat field about a mile from Worcester, Mass., on the road to New Worcester.] then the drug consists of straight, few branched stalks, from six to twenty-four inches long. If culled from moist situations along the banks of streams, the plants are more robust, branched and bushy.
Farmers often gather little lots of lobelia and then the entire plant is sold. Root and herb collectors on the contrary understand that the seed commands a better price than the herb, and they thresh out the seed, break or chop up the stalk, and sell the seed separately. Thus it happens that the larger part of the lobelia herb of commerce is devoid of seeds, and is in a broken condition. As a rule, the leaves and capsules are of a green color, the upper capsules being especially verdant.
No substitution for Lobelia inflata herb or adulterant is gathered, of which we are aware, nor is any probable. Lobelia cardinalis and Lobelia syphilitica are such different appearing plants they would be easily detected, and the other native and more closely allied species are so small and mostly rare that it would not be profitable to collect them.
According to the Pharmacographia the drug used in England is mostly imported packed in ounces. [The herb found in commerce is in the form of rectangular cakes, 1 to 1 3/4 inches thick, consisting of the yellowish green chopped herb, compressed as it would seem while still moist, and afterwards neatly trimmed. The cakes arrive wrapped in paper, sealed up and bearing the label of some American druggist or herb-grower."—Pharmacographia, 1879, p. 399.]
Some writers assert that the root of Lobelia inflata is employed. This is a mistake, and first made by confusing Lobelia syphilitica with this plant. The root of Lobelia syphilitica was employed before Lobelia inflata was known to medicine, but the root of Lobelia inflata has never been used.
All parts of Lobelia inflata contain an acrid alkaloid (see Constituents, page 73,) which produces a painful irritation upon inhaling the dust of any portion of the plant. All parts of the herb, and the seed, produce an acrid biting sensation on the tongue, and a sharp tobacco-like impression in the throat and fauces. The milky juice of the green plant is intensely acrid, owing perhaps to the more soluble condition of the alkaloid. This juice is so violent that an amount so small as to refuse to affect a balance sensible to the one-thousandth part of a grain, produces a sharp tingling sensation upon the tip of the tongue. Upon drying, this juice becomes very much modified, but not by the escape of a volatile alkaloid.
The first published description of Lobelia inflata [Account of Indigenous Vegetables.—Cutler, 1785.] states that the leaves if chewed "produce giddiness and pain of the head, with a trembling agitation of the whole body," and this sentence with little variation has passed through a multitude of works on materia medica. [The original description of a drug seems to be authority with many writers who neglect to give proper credit to the real author, and, who seem not to display much personal knowledge of the subject.] It has not been our experience to note a giddiness of the head, the sensation with us is simply a tobacco-like irritation until nausea, headache and vomiting occur, and this is the report of others, who we know to be familiar with the drug.
Lobelia Seed.—This drug presents a deep brown color in mass. It consists of minute, almost microscopic seed. Their actual size is about 1-60 of an inch in length by 1-240 of an inch in diameter. The typical seed is oblong, rounding at the ends, and cylindrical. Sometimes they are nearly round, however.
The average number of seeds in a capsule is between 450 and 500. It requires 2500 seed to make one grain in weight. [Thus, a pound will contain 17,500,000 seed. The business firm with which the writers are connected purchased recently in one lot 2000 pounds of lobelia seed. By our calculation this amount contains the enormous number of 35,000,000,000 individual seed.] Upon microscopic examination, each seed is shown to be a beautiful object, bright and glistening, the surface being a corrugated ridge-like network, of which figure 131 is a representation.
 Lobelia seeds are odorless, but upon handling them a fine dust rises that is very irritating when inhaled. They possess the acridity of the plant in an intensified degree, and were considered by the Thomsonians to possess one-half more strength (emetic) than the powdered leaves.
Lobelia seeds are odorless, but upon handling them a fine dust rises that is very irritating when inhaled. They possess the acridity of the plant in an intensified degree, and were considered by the Thomsonians to possess one-half more strength (emetic) than the powdered leaves.
Lobelia seed have never been officinal, but are in good demand in the American drug market, and, extensively employed by Eclectic physicians who consider that the preparations of the seed are more uniform and reliable than those of the herb. Our experience is to the same effect.
No adulterations or sophistications are known to us, although often fragments of the leaves and capsules are present, not being separated by sifting through fine enough sieves. The commercial term for the drug free from this chaff is "clean lobelia seed."
The corrugated surface of the seed is a characteristic of the species of Lobelia, and would serve to individualize them. It would be possible to substitute the seed of other species, Lobelia syphilitica, and perhaps Lobelia cardinalis. We made a careful comparison under a microscope of the seed of Lobelia syphilitica and Lobelia inflata and were unable to note any difference either of size or marking.
We are not aware that the substitution is ever made by collectors, but it could be done with profit to them as the Lobelia syphilitica produces seed in abundance and is a common plant and easily collected.
Fortunately, however, the plants are so different in all appearances that ignorant collectors have no idea that they are at all similar and the substitution is not suggested to them.
![]() MICROSCOPIC STRUCTURE OF LOBELIA INFLATA.—(Written for this publication by Robt C Heflebower, M. D.)—Transverse and longitudinal sections of the stem of the plant show first the epidermis. (See fig. 132, plate xxxv, and fig. 136 following page.) This consists of a single layer of cells, and supports the hairs found upon the surface of the stem. Beneath this layer are several other layers of cells, (a. figures 132 and 136,) mostly oval upon transverse, and elongated upon longitudinal section. The cells of this layer are not all closely approximated, but there is a small space existing between some of them, whilst others are intimately joined. The latter is usually the case.
MICROSCOPIC STRUCTURE OF LOBELIA INFLATA.—(Written for this publication by Robt C Heflebower, M. D.)—Transverse and longitudinal sections of the stem of the plant show first the epidermis. (See fig. 132, plate xxxv, and fig. 136 following page.) This consists of a single layer of cells, and supports the hairs found upon the surface of the stem. Beneath this layer are several other layers of cells, (a. figures 132 and 136,) mostly oval upon transverse, and elongated upon longitudinal section. The cells of this layer are not all closely approximated, but there is a small space existing between some of them, whilst others are intimately joined. The latter is usually the case.
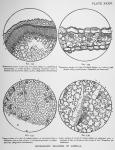 Lying to the inner side of these outermost strata is the parenchyma of this portion, (p. figures 132 and 136.) It consists of numerous cells, elliptical in outline, arranged in from five to seven layers around the entire stem In many places these cells appear irregular in form, this irregularity being caused by pressure from adjoining cells.
Lying to the inner side of these outermost strata is the parenchyma of this portion, (p. figures 132 and 136.) It consists of numerous cells, elliptical in outline, arranged in from five to seven layers around the entire stem In many places these cells appear irregular in form, this irregularity being caused by pressure from adjoining cells.
The woody structure of the plant (b. figures 132 and 136) is comparatively thick, and forms distinct medullary rays and interspaces.
Towards the pith, at the center of the stem, are the different vessels of the plant, the lactiferous tubes (c. figures 132 and 136) and the tracheae. The structure at this portion is complicated, but the tracheae are easily seen. They consist chiefly of spiral and annular vessels, the markings of which are very beautiful. Some pitting of the cell walls is also present. The lactiferous tubes are of the articulated variety, and by means of branches extending from one longitudinal tube to another, form a reticulated anastomosis.
The pith, (d. figures 132 and 136,) situated at the center of the stem, consists of a mass of loosely arranged cellular tissue, formed of numerous elongated cells, which, by transverse section, give an oval, a polygonal or a round outline.
There are also other epidermal structures besides those already mentioned. These are the hairs found upon both the stem and the leaf, the stomata of the leaf and the arrangement of the epidermal cells around such hairs and stomata.
The hairs upon the stem are simple and compound. The simple hairs project directly from the epidermis of the stem, and are unicellular of an elongated conical shape, having a base or attached portion, and an apex or free portion.
The compound hairs (see fig. 127, page 63) arise by a single trunk, from which project several branches. These branches resemble the simple hairs.
The epidermes of both surfaces of the leaf present cells bounded by irregular outlines and hair structures. The cells of the upper surface (see figure 133) are larger, and their walls thicker, than those of the under surface. The same is also true of the hairs of this surface. [The apparent contradiction to this statement of our figures, number 133 and 134, is from the latter being more highly magnified.] The under surface (see fig. 134) presents in connection with the simple epidermal hairs and cells, numerous stomata, (see s. fig. 134.) Each stoma is widely elliptical in shape, and consists of a pore or longitudinal slit, and the guard or stomatal cells which bound the pore. Outside of the guard cells are several epidermal cells surrounding the stoma, the subsidiary cells of the stoma. The base of the hair is likewise surrounded by a similar cluster of cells, the subsidiary cells of the hair.
 A transverse section of a leaf of Lobelia inflata (see fig. 135) presents the epidermis of each surface beneath the cuticle, and the parenchymatous structure between the two epidermal layers. The cells of the parenchyma are filled to a greater or less extent by chlorophyll granules.
A transverse section of a leaf of Lobelia inflata (see fig. 135) presents the epidermis of each surface beneath the cuticle, and the parenchymatous structure between the two epidermal layers. The cells of the parenchyma are filled to a greater or less extent by chlorophyll granules.
The pollen grains are ovoidal in form and resemble a wheat grain, having a longitudinal slit on one side dividing the grain into lateral halves.
CONSTITUENTS.—Lobeline.—The characteristic principle of Lobelia inflata is an acrid, irritating alkaloid, that pervades all parts of the plant; most easily obtained from the seed. It is known as lobeline.
It exists in combination with an unimportant vegetable acid. If freed while in contact with other constituents of the plant the alkaloid decomposes in a short time. If heat is applied to an aqueous solution of the natural constituents, this decomposition occurs rapidly and the alkaloid soon disappears. [This fact was well known to the Thomsonians. They used but little heat, and throughout their literature we find constant reference to the loss of strength by boiling. Indeed, they wisely prefered to give both the herb and seed in substance. Empiricism, demonstrated what chemistry supports.] Heat applied even to an alcoholic tincture accomplishes the rapid destruction of the alkaloid.
In a recent experiment whereby we evaporated in a close still the alcoholic tincture of fifty pounds of Lobelia seed, and extracted the residue with acidulated water, having neglected to add the acid to the alcohol, most of the lobeline perished. In another experiment, by an oversight, heat was applied to an aqueous solution of the alkaloid, while it was associated with other constituents of the plant and the alkaloid entirely disappeared. [Here again the Thomsonians learned from experience. They used acetic acid to make their most stable preparations.]
History of Lobeline.—Prof. S. Cohoun, 1834, [Prof. S. Calhoun, M.D., was Professor of Materia Medica in Jefferson Medical College, Philadelphia, at the time he wrote this paper.] made the first examination of Lobelia inflata. He obtained by means of acidulated alcohol, a colored liquid that he took to be the characteristic principle, which however was simply a crude extract containing a salt of the alkaloid. He described it as follows: "The active principle of this plant is a brown, molasses-like fluid."
Prof. Wm. Procter, Jr., 1838, [Am. Journ. Pharm., 1838, p. 98, illustrated.] made Lobelia inflata the subject of his thesis. This was the first creditable chemical investigation of the plant. By a number of experiments he fairly demonstrated the presence of a volatile oil destitute of acrimony (exp. 4,) an alkaline body, soluble in ether, (exp. 10 and 11,) [He erroneously gives to this a strong odor. The odor was due to impurities.] which is capable of forming salts with acids, (exp. 12.) [In 1840, (Am. Journ. Pharm., p. 280,) Prof. Procter examined Lobelia cardinalis, obtaining an impure alkaloid, of a bitter taste. Informed salts with acids.]
Again, 1841, [Am. Journ. Pharm., 1841, p. 1.] Prof. Procter reconsidered the subject and obtained the alkaloid lobeline as a yellow, oily liquid, but he states, "if the process of purification were repeated, there is little doubt but that the lobeline would be obtained perfectly colorless."
Reinsch, 1843, [Pharmacographia, p. 400.] obtained a substance that he called lobeliin, but which was not a definite body.
W. Bastick, 1851, [Pharmaceutical Journ. and Trans., 1851, p. 270.] attempted to clear up the lobeline record, but was far from being successful, and added little if anything thereto. He obtained Mr. Procter's impure alkaloid by employing Liebig's process for making hyoscyamine.
Mayer, 1865, [Proceedings of the American Pharmaceutical Association, 1865, p. 211.] in considering the "Principal Reactions of the Medicinal Volatile Bases" records the action of lobeline, classing it with the volatile alkaloids known at that day. In our opinion lobeline is not a member of the class (volatile) he investigated.
In 1871, [Pharmaceutisches Central-Blatt, No. 31, July 5, 1843.] Enders extracted lobelia with alcohol and distilled the liquid in presence of charcoal, washed the charcoal with water and extracted it with alcohol which yielded warty tufts, slightly soluble in water, brown, acrid, and uncrystallized. Soluble in chloroform and ether. He gave it the name Lobelacrin, but we find it to contain the substance we designate as inflatin and a little of the alkaloid lobeline.
W. D. Richardson, 1872, [Inaugural Address, Am. Journ. Pharm., 1873, p. 392.] found that upon exposure, lobeline underwent a change whereby it became insoluble in water and refused to form salts, but the nature of the alteration was undecided.
Mr. W. H. D. Lewis, 1878, [Pharm. Journ. and Trans., London, 1878, p. 561. Mr. Lewis was a member of the Pharmacy class of the University of Michigan at the time he wrote the paper.] reviewed the literature on the lobeline subject, and suggested a modification of preceeding processes, whereby he obtained lobeline of a honey-like consistence and light yellow color, hut evidently impure, as it had "a somewhat aromatic odor." He decided that lobeline exists in the plant in combination with lobelic acid, and affixed to this salt the name lobeliate of lobiline, but, this substance, (whatever it may be,) had previously been obtained by Procter.
Dr. H. Rosen, 1886, [An Inaugural Dissertation, University of Dorpat, 1886, communicated to the Am. Journ. Pharm., 1886 392. His paper was on Lobelia nicotianaefolia, but he states, "the same two alkaloids were also obtained from Lobelia inflata."] obtained lobeline by making a benzin solution from the acrid infusion, and another alkaloid as he thought by after treatment of this liquid with chloroform. He decided that the latter alkaloid presented striated prisms. His investigations were evidently performed with small quantities from which possibly he failed to separate impurities.
Résumé.—Thus it is that, although much time and attention have been given to the lobelia constituents, the result is far from satisfactory. In our opinion, the chemistry of the subject is yet obscure. We have followed the various processes and obtained the acrid alkaloid, amorphous, colorless, intensely active, one drop of its solution immediately vomiting a strong man, but we have not crystallized either the pure alkaloid or a salt of it. We obtained crystals from the impure alkaloid lobeline, as others had and for some time accepted that they were the corresponding salts, but further (recent) examinations enabled us lo eliminate the crystalline material entirely, leaving the alkaloid as an amorphous product. [We simply state that we were misled. The crystals that we obtained were not of lobeline, but an impurity that intimately accompanies it and crystallizes more easily under the influence of acid liquids. Our crystals compare too, with Procter's description.] That we were for a while deceived is evident, that others may also have been misled is possible. For the present we shall simply call this crystalline substance inflatin, [We dislike to affix a name to a body that is so obscure in its classification as this now is. We find also that the various forms of the word lobelia is entirely monopolized. Hence, we reluctantly select inflatin for want of a better name.] and are led to make this introduction before referring to the preparation of lobeline.
Preparation of Lobeline.—Extract the oil from powdered lobelia seed, by means of benzine, and dry the residue. Then acidulate the dry powder with a mixture of acetic acid one part, alcohol nine parts, and pack firmly in a glass percolator. Exhaust with a menstruum made of acetic acid one part, alcohol twenty parts. Evaporate the liquid, and when cold, add water enough to make a thin syrup, and extract the alkaloid from it by means of ether, adding cautiously ammoniac [Some use magnesia, thinking that ammonia decomposes the alkaloid. Any alkali and heat will do so, but dilute ammonia in presence of ether does not alter it in appreciable amounts. Magnesia does not entirely decompose the salt (acetate) and a free alkali is necessary.] to slight alkaline reaction. The ethereal liquid is then to be decanted, evaporated in presence of water that has been previously acidulated with acetic acid to excess. The watery layer is cooled, separated from overlying oil, filtered, and again extracted with ether to which ammonia is again cautiously added to slight excess. This ethereal liquid will be colorless (if not so repeat the operation) and it contains the alkaloid lobeline. It has been supposed to contain only the alkaloid, but, in addition there is a volatile oil and inflatin.
If this ethereal solution is evaporated, a colorless glassy layer remains, of a strong odor, and which turns yellow and even brown upon exposure. It is partly soluble in acidulated water, [It does not necessarily follow that because this body was once entirely dissolved in acidulated water, it will completely redissolve after being dried.] yielding the alkaloid, mixed with various amounts of the associated impurities. It dissolves in alcohol, ether and chloroform, but only incompletely in benzol and carbon disulphide.
If the ethereal solution is evaporated in contact with acids (excepting acetic acid) an amorphous layer usually interspersed with crystalline formations remains. These crystals we formerly took to be salts of lobeline, even drawing fig. 138 under the impression that it was a sulphate. If this crystalline layer be extracted with carbon disulphide, [We think that former investigators failed to break up this mixture by using ether and alcohol only as solvents. These liquids dissolve the entire associated products, and acid water will also do so to an extent, although pure inflatin is insoluble in water.] the crystals disappear [See inflatin, p. 76.] and the acrid material remains. If now, the residue (a salt of lobeline} be exposed to the dry atmosphere for a few days, it becomes odorless from escape of the volatile oil. Then, it will dissolve in water, especially if slightly acid, and after filtration can be extracted colorless and as we now believe pure, by sulphuric ether in connection with a slight excess of ammonia. [We make no claim to originality in the method of making lobeline. Our process differs somewhat from others it is true, but, perhaps not materially. The aim is to divest the seeds of their oil, extract the alkaloid in stable condition and eliminate impurities without the application of more chemistry than is necessary.]
Properties of Lobeline.—Lobeline is alkaline in reaction, colorless, odorless, soluble in alcohol, chloroform, ether, [Wittstein in his Organic Constituents of Plants states that lobeline is insoluble in ether. This is a mistake.] benzol, carbon disulphide, and somewhat soluble in water. We have not succeeded in crystallizing it. It is not hydroscopic (Wittstein contra.) In pure condition lobeline can be exposed to the air for days, and is probably permanent. We evaporated by exposure, a solution in water rendered strongly alkaline by ammonia, [It is stated that alkalies destroy lobeline at once. This is incorrect.] which changed to yellow, showing some decomposition, but which retained all the sensible properties of the alkaloid, remaining very acrid and being a violent emetic.
Lobeline turns red with sulphuric acid, yellow with nitric acid and dissolves colorless in hydrochloric acid. Heated with sulphuric acid it turns black; with nitric acid evolves the usual vapors. of nitric oxide, with formation of a yellow liquid; and hydrochloric acid evaporates from it unchanged.
Salts of lobeline are very soluble in water and those we have examined dissolve in alcohol and ether, but very slightly (excepting the acetate) in carbon disulphide. [This solvent which seems to have been overlooked by others enabled us to purify the crude lobeline as already stated and as further explained under inflatin.]
From moderately strong aqueous solutions of the salts of lobeline, alkali precipitates the alkaloid, white, flocculant, amorphous and odorless. This precipitate dries to a glassy layer that will powder white, [This differs from statements of others, who describe it as an oily liquid.] but this must be cautiously performed as minute amounts of the dust excite violent irritation of the nostrils, air passages and lungs, equal to, if not more intense than veratrine.
All the alkaloidal reagents precipitate lobeline from aqueous solution of its salts.
We have as yet failed to crystallize salts of pure lobeline, but we think that such a positive alkaloid will furnish crystals under proper conditions. [Sulphate of lobeline is quoted in commerce. We see no reason for presuming that if demanded in quantities it should not be crystallized. We also think that manufacturers who have a demand for the alkaloid should have been able to exclude the crystalline substance that we have found to accompany it.]
Lobeline and its salts are among the most powerful of emetics, and extremely small amounts of the solution of the colorless alkaloid, (one drop being placed on the tongue) immediately vomited those to whom we administered it. There was no unpleasant after effect (see medical properties.) In the crude condition, as former investigators have obtained it from ethereal solution (even color" less as we made it) decomposition occurs and it rapidly darkens.
Résumé.—The alkaloid lobeline has evidently been impure as heretofore described, and may not be pure as we obtain it. Others state that it is yellow and has an odor; this certainly is erroneous for we produced it colorless and odorless. Others have obtained what was considered crystalline salts; we also formerly thought this easy, but found the crystalline material to be an impurity, to which we can find no previous reference. It has never been analyzed, but, if our present line of manipulation is successful, further remarks will follow, and a combustion made by recognized authority.
Having considered the most prominent constituent of lobelia, we shall now pass to the most characteristic principle which as before stated we have for descriptive purposes designated as inflatin.
Inflatin.—This substance exists ready formed in lobelia herb and seed, and maybe extracted together with the fixed oil and chlorophyll by means of carbon disulphide. Since the oil passes with the inflatin through most solvents and holds it in solution when the other solvents are evaporated, it is not feasible to separate inflatin from the extracted oil, although, we have obtained it by saponifying the oil and separating the soap.
Inflatin has certainly been obtained by the investigators who produced crude lobeline, beginning with Prof. Procter, but owing to its intimate association with that alkaloid, and with the volatile oil of the plant, and to its refusal to crystallize while associated in this manner it has been overlooked. [Even if it has crystallized, the solvents formerly employed redissolve both it and the associated principles.]
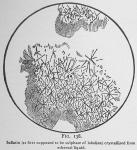 The glassy layer first obtained in the evaporation of lobeline from the ethereal liquid, if moistened with acid solutions will upon drying assume a partly crystalline condition. This led us (see page 75) to conclude that the salt of lobeline had crystallized, and figure 138, as before stated was drawn under the supposition that it was a sulphate of lobeline. These crystals with varying conditions assume different forms, and hence, we were more easily misled when we used the several acids.
The glassy layer first obtained in the evaporation of lobeline from the ethereal liquid, if moistened with acid solutions will upon drying assume a partly crystalline condition. This led us (see page 75) to conclude that the salt of lobeline had crystallized, and figure 138, as before stated was drawn under the supposition that it was a sulphate of lobeline. These crystals with varying conditions assume different forms, and hence, we were more easily misled when we used the several acids.
Preparation of Inflatin.—Evaporate in thin layers the ethereal solution of crude lobeline (obtained by process on page 75) adding hydrochloric acid to slight excess. To the sticky product before completely dry, add a few drops of carbon disulphide, [This leaves the hydrochlorate of lobeline.] and after flowing it about decant the solution into a shallow vessel. Repeat the operation with successive portions of carbon disulphide, and mix the liquids. It is best, if working small amounts, to allow the preceeding portion to evaporate each time before adding the other.
The final product will resolve itself in a few hours into small white warty aggregations, perhaps (if very impure) imbedded in a viscid, tenacious, more or less yellow semi-liquid. These globules are inflatin, destitute often of crystalline form because of the pressure of the surrounding medium. Occasionally an isolated globule like a. fig. 139 will resolve itself into a fragment like b. fig. 139, and we have seen these globules under the microscope become crystalline strata without change of shape.
Carefully drop carbon disulphide on this layer and decant it at once into a clean glass as soon as it has taken up the globules, which will be before the yellow substance dissolves. As the carbon disulphide evaporates crystalline nodules will form. The crystals do not form as distinct, however,as if the product is redissolved in pure benzol and evaporated.

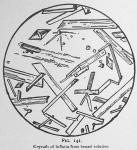
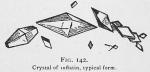
 Thus purified the crystals may appear like figures 140, 141, 142 and 143, dependent on the evaporation and depth of the liquid. Where the liquid is very thin, we observe a display like figure 140; if deep they will appear like figure 141; if deep enough to permit the typical crystal to form, they will mostly be diamond shaped [The goniometer must be used to determine their exact crystalline form. They appear to us as our artist represents them.] as shown in figure 142.
Thus purified the crystals may appear like figures 140, 141, 142 and 143, dependent on the evaporation and depth of the liquid. Where the liquid is very thin, we observe a display like figure 140; if deep they will appear like figure 141; if deep enough to permit the typical crystal to form, they will mostly be diamond shaped [The goniometer must be used to determine their exact crystalline form. They appear to us as our artist represents them.] as shown in figure 142.
 Since we have discovered the characteristics of this material, we have obtained it easily as follows: Abstract the greenish oil from powdered lobelia seed by benzine, stopping the percolation when the percolate ceases to pass of a green color, (this abstracts much inflatin also.) Dry the magma and extract it by means of carbon disulphide. Evaporate the carbon disulphide and cool the residue. It will crystallize to a magma of inflatin and a fixed oil. Place on bibulous paper and warm it, the oil is absorbed and the inflatin can be purified by crystallization.
Since we have discovered the characteristics of this material, we have obtained it easily as follows: Abstract the greenish oil from powdered lobelia seed by benzine, stopping the percolation when the percolate ceases to pass of a green color, (this abstracts much inflatin also.) Dry the magma and extract it by means of carbon disulphide. Evaporate the carbon disulphide and cool the residue. It will crystallize to a magma of inflatin and a fixed oil. Place on bibulous paper and warm it, the oil is absorbed and the inflatin can be purified by crystallization.
Properties of Inflatin.—Inflatin is pure white and from carbon disulphide tends to form nodules of a crystalline structure or in great crystalline plates. The various modifications of the crystals are shown by figures 140, 141, 142 and 143. The typical crystal is diamond shaped and perfectly transparent.
Inflatin is odorless, tasteless and refuses to unite with acids or alkalis. It is insoluble in water or glycerin, but soluble in carbon disulphide, benzol, chloroform, ether and alcohol in the order we have given. Sulphuric acid does not affect it, even the smallest crystals remaining sharp and distinct. Hot sulphuric acid decomposes it with formation of a black liquid.
Cold nitric acid has no action upon it, but developes the forms and angles of a crystalline layer under the microscope in magnificent distinctness, the centers of each crystal being pure white, and the ends jet black as shown by figure 143 a, developed from a slide of which 143 b is a part without the nitric acid. Upon heating with nitric acid inflatin melts without change of color, and upon evaporation of the acid, and resolution in benzol, crystallizes as before.
 Upon boiling inflatin with Fehling's solution it turns brown, then black, but does not reduce the copper and does not dissolve.
Upon boiling inflatin with Fehling's solution it turns brown, then black, but does not reduce the copper and does not dissolve.
Inflatin melts at 225° F , and at a lower temperature cools to a mass of crystalline structure.
Résumé.—From the preliminary examination that we have given this substance, we conclude that it is either a stearoptene or a vegetable wax, probably the former. Perhaps in mechanical suspension it produces the milky juice of the plant, but we did not discover it in time to examine the juice of the herb during its season. It is evidently of no medicinal importance, and, is of interest we think simply because of its association with the other constituents of lobelia.
Volatile Oil of Lobelia.—Lobelianin.—All parts of the herb of fresh lobelia are pervaded by a volatile oil of a strong pungent odor, but with little taste and no acridity. It was described by Procter, (see p. 74,) 1838, who found that the tincture of lobelia, or the herb, distilled with water gave a distillate of a peculiar odor. Fareira, 1840, gave it the name Lobelianin, and stated that it had an acrid taste, but, Procter, 1842, decided that he was mistaken on this point, and, our investigations support Prof Procter. [We made a careful examination, distilling water from quantities of the herb, both fresh and dry, and we used the utmost care to avoid the passing over of spray with the vapor The product gave simply (from the green herb) a volatile oil that could be separated by sulphuric ether, but it docs not accumulate in amount sufficient to separate from the distillate unless the temperature be very low.]
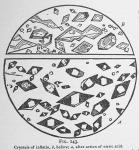
 If a small amount of water be destilled from a large quantity of the dry herb, (Pereira and Procter used the dry.) and the destillate be reduced to about the freezing point of water, it deposits groups of transparent crystals, which do not redissolve when the water is warmed. Upon dissolving them in appropriate solvents (any of the usual solvents for volatile oils) and evaporating the menstruum, this oil crystallizes in large groups of flat, transparent plates that do not often radiate from a common center They cover the slide and are nearly parallel connected by oblique plates, but not often in stellar groups, (see fig. 144.)
If a small amount of water be destilled from a large quantity of the dry herb, (Pereira and Procter used the dry.) and the destillate be reduced to about the freezing point of water, it deposits groups of transparent crystals, which do not redissolve when the water is warmed. Upon dissolving them in appropriate solvents (any of the usual solvents for volatile oils) and evaporating the menstruum, this oil crystallizes in large groups of flat, transparent plates that do not often radiate from a common center They cover the slide and are nearly parallel connected by oblique plates, but not often in stellar groups, (see fig. 144.)
Upon heating crystals of lobelianin [Perhaps this name is inappropriate and should not be applied to a concrete volatile oil. However, it was first given by an authority we all respect and it has precedence.] suspended in water it melts at a temperature of 160° F., and if melted on a glass surface it quickly evaporates without residue, evolving the pungency familiar to those who know the recent distillate.
It slowly evaporates upon exposure to the air and disappears.
Sulphuric and nitric acids dissolve it and upon heating a slide of crystals to which a drop of nitric or sulphuric acids had been respectively added, the nitric acid evaporated without apparent change, while the sulphuric acid blackened and evolved empyreumatic vapors. It retains its crystalline form in ammonia water and liquor potassa.
We could not determine if more than one oil is obtained by the act of distillation, but, it is probable that such is the case.
We endeavored to obtain the substance we have called inflatin, by oxidation of this oil, but failed, although it is apparent that some constitutional difference exists in the volatile oil of fresh lobelia and that of dry. The oil of fresh lobelia did not crystallize in our hands.
Has Lobelia a Volatile Alkaloid?—Prof. Procter, 1838, [American Journal of Pharmacy, 1838, p. 104, experiments 4, 5 and 6] found that both tincture of lobelia and the herb, with water, upon distillation gave a distillate of a peculiar odor but destitute of acrimony. Pereira, 1840, [Elements of Materia Medica, vol. ii., 1846, p. 385, (and preceding edition.)] stated that it had in addition an acrid taste, which Procter, 1842 [American Journal of Pharmacy, 1842, p. 4.] decided was a mistake. Bastick, 1851, [Phar. Journ. and Trans., 1851, p. 270.] states that "lobeline is volatile."
We made a careful examination, distilling water from quantities of the fresh herb. We used the utmost care to avoid the passing of undistilled liquid with the vapor, and failed to obtain either an alkaloid or an acrid distillate. [The herb for these experiments was gathered to order and selected plant by plant There was no foreign substance present and the lobelia was prime.] The product was of strong odor, from it sulphuric ether dissolved the oil, but there was no trace of acridity or of an alkaloid. Then we used dry fresh lobelia in ten pound lots, with water, and with water that was made alkaline with caustic potash. In both cases the distillate was free from acridity and refused to affect any alkaloidal reagent.
We made a solution of pure sulphate of lobeline, rendered it alkaline with caustic potash, and distilled it to one-third. The distillate gave evidence of decomposition products, but no lobeline came over. [The neck of the retort was plugged near the retort with a strainer of linen to retain the spray. The neck was inclined to throw the condensed liquid back into the retort. Thus only the vapor passed to the condenser. In the large still with the herb, the exit for vapor extended upward 55 feet to the condenser and a spray could not pass over.]
We therefore conclude that lobelia does not contain a volatile alkaloid, and that lobeline is not volatile. There is no reason that we can see to suppose that the alkaloid lobeline is chemically related to the alkaloid nicotine. That they have been associated is probably from the unfortunate name for lobelia, Indian tobacco, and the fact that the plants and alkaloids resemble in taste, and that both are emetic.
LOBELACRIN. (So called.,—Enders, 1871, [Pharmacographia, p. 400.] obtained a substance that he named lobelacrin. It was produced by exhausting lobelia with alcohol, adding charcoal and distilling. The charcoal was washed with water, treated with boiling alcohol, the alcohol evaporated a:id the residue extracted with chloroform Upon evaporation of the chloroform "warty tufts" of a brown color were obtained. This, Enders named lobelacrin. Lewis considered it perhaps a lobeliate of lobeline. We consider it a mixture of the oil (fixed) of lobelia, the substance that we have called inflatin, a brown resin, some lobeline and coloring matter. According to our examination, it is really a mixture of such substances as are extracted from lobelia by alcohol, and having refused to dissolve in water are soluble in chloroform. It will be evident to the reader that this process certainly cannot separate the oils, wax and like bodies. That an organic acid is present is also probable.
Fixed Oil of Lobelia.—Lobelia seed contains thirty per cent, of non-volatile oily matters. The true fixed oil of lobelia is bland and non-acrid. As usually obtained, even by expression, it is acrid from contaminations. Menstruums that dissolve the oil also dissolve the chlorophyll, hence it has a green color as extracted from powdered seed. Pure fixed oil of lobelia has never been used in medicine and would be of little value.
An impure oil is a favorite with Eclectic physicians, who use it alone and associated with other substances. It is a constituent of Compound Stillingia liniment, [See unofficial pharmaceutical preparations of lobelia, to follow.] an excellent remedy, which in our opinion depends mainly upon this impure oil, which is simply a syrupy extract of lobelia seed, made with stronger alcohol acidulated with acetic acid.
Other Constituents of Lobelia.—There is a characteristic brown resin, coloring matters, and the usual constituents of plants. If the resin in alcoholic solution be precipitated by water even in presence of acid water, it carries with it a large amount of lobeline. This we thought to be a distinct alkaloid, but became assured after purification, that it was simply lobeline. [Many resins have strong affinities for alkaloids and other constituents of plants They act somewhat like animal charcoal, carrying them from solution and holding them tenaciously.]
Continued on next page.
Footnotes.
* Matthias de Lobel (Matthias de 1'Obel as the name is originally spelled) should be classed among the early English botanists. He was born in 1538 at Lisle in the north of France and was educated at Montpelier in the south of France, and traveled over Italy, France, Germany, finally settling near London. By profession he was a physician, and at one time he was physician to William, Prince of Orange. His chief reputation, however, is as a botanist, this study seeming to occupy most of his time. In 1570 he published at London a small work entitled "Stirpium Adversaria" which professed to investigate the botany and materia medica of the ancients, especially of Dioscorides.
A second edition of this work in 1603 contained an addition on new remedies, rare plants, etc., and in this work the first glimpse of a natural system of classification can be seen. It was necessarily very crude and imperfect, and consisted merely in grouping together such plants as seemed to accord in appearances or habits, without however defining the groups, or making any allusion whatever to the system. Some of the groups such as leguminous, grasses, etc., are quite natural and have continued to the present day, others, as could be expected, are very incongruous. The work was printed in Latin and on this account was little known to the common people.
For the times in which he lived, Lobel was a most learned man in botany and the leader in this science He styled himself (on one of his title pages) "botanist to king James I.," which has no doubt been the source of the erroneous statement published in several encyclopaedias that he was "physician to king James I."
Lobel died in 1616, aged 78 years.
**George Clifford was a wealthy banker, who resided in Amsterdam in Holland at the time of Linnaeus. He was not a close student of natural science, but having a liking in this direction and abundance of means he established an extensive private garden, obtaining the most rare and expensive exotics.
Becoming acquainted with Linnaeus, who was at that time in straitened circumstances, and recognizing his talents, Clifford employed him to study and superintend his garden, giving him a liberal salary.
For the first time in his life, Linnaeus had now leisure and means to pursue his studies, unembarrassed with the necessity of struggling for a living and the result was the great systematic works that have made his name famous.
For three years Linnaeus remained at Amsterdam and published the Hortus Cliffortianus, a magnificent work, enumerating all the plants that were in the garden or collection of his patron. Some idea of the wealth and liberality of George Clifford may be obtained from the fact that this expensive work, of over 500 folio pages and numerous plates, was only distributed gratuitously to his friends and correspondents.
A genus of shrubs, Cliffortia, of the Cape of Good Hope, commemorates his name.
Drugs and Medicines of North America, 1884-1887, was written by John Uri Lloyd and Curtis G. Lloyd.

