BY FRANK L. SLOCUM, PH.G.
From an Inaugural Essay.
The microscopical structure of the rhizome of Sanguinaria has not yet, to my knowledge, been figured, and is only briefly mentioned by De Bary in his "Vergleichende Anatomie," p. 209, 450. Hence a microscopical examination has been made.
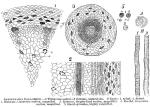 Fig. 1 represents a cross-section of the rhizome, showing the general arrangements of the fibrovascular bundles which are situated in a double circle three-fourths of the distance from the center to the exterior.
Fig. 1 represents a cross-section of the rhizome, showing the general arrangements of the fibrovascular bundles which are situated in a double circle three-fourths of the distance from the center to the exterior.
Outside of the xyleme the parenchyme is rather compressed; the 8 or 10 external rows of cells are generally quite devoid of starch, and contain a few resin cells, gg. The fibrovascular bundles in the outer circle are composed of about 12 vessels each, shortly jointed, and their course is exceedingly difficult to trace. They are all of one class, namely, pitted vessels; the sieve tubes are few, and nearly all situated in the outer portion of the fibrovascular bundles. The fibrovascular bundles in the inner circle are smaller, the vessels are longer and their course is quite easily traced; the sieve tubes are in the same position as in the outer row of bundles.
Inside of the circle of fibrovascular bundles, and between them, is loose parenchyme, filled with starch; the large cells, ee, containing the red juice, are shown with the juice dried and adhering to the cell walls.
According to De Bary, (loc. cit.), laticiferous ducts are absent in the Sanguinaria, having in their place large thin-walled cells, filled with red juice. In only one specimen out of nearly 50 examined were found spiral ducts in the rootlets and inner circle of the fibrovascular bundles.
Fig. 2 represents a longitudinal section of the rhizome, the structure of which may be understood from the above explanation of the cross-section.
Fig. 3 represents a transverse section of a rootlet; the vessels are seen to be closely aggregated in the center, surrounded by sieve tubes, which, as they become more removed from the vessels, are of somewhat, but slightly, increased diameter. Outside of the nucleus sheath the structure consists of parenchyme, flattened and elongated, and containing resin cells.
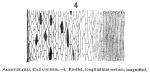 Fig. 4 shows a longitudinal section of a rootlet, which is understood by the above description of the transverse section.
Fig. 4 shows a longitudinal section of a rootlet, which is understood by the above description of the transverse section.
Fig. 5 shows the starch granules highly magnified, probably two-thirds of the granules being of the size of 1-ab, while the remainder are of the size 1-cd.
The granule 1-a measures 6.022 mm., while the smallest granules measured 0.0032 mm. 2 shows the appearance of a granule under polarized light.
A represents a transverse section of rhizome, of natural size.
B 1 and 2 represent two ducts much magnified, 1 being a spiral duct from a rootlet, 2, a dotted duct from the rhizome.
The external layer of cells in both rhizome and rootlet do not differ materially from the others, being only slightly flattened.
Chemical Examination.—Four pounds (avd.) of carefully selected rhizome were reduced to powder No. 50 and exhausted with stronger alcohol; the alcohol was removed by distillation, leaving a soft dark red extract, weighing 3/4 pound (avd.). The extract was then mixed with half a gallon of acidulated water (water 48 parts, acetic acid 1 part), which precipitated the resin, leaving a blood-red solution; the resin was removed by nitration, and thoroughly washed with distilled water, in which it is nearly insoluble, dried and weighed; the yield was 985 grains of resin, (a); the filtrate was marked (6).
Examination of Resin (a).—The resin is of a dull pale red color, slightly sternutatory, has an acrid taste, and is of waxy consistence. An examination was made by the following scheme to ascertain its nature:
| The resin was dissolved in boiling alcohol and the solution cooled, when a precipitate was formed; separated by a filter. | ||||||
| Filtrate.
Treated with alcoholic solution of acetate of lead, which produced a slight brown precipitate; separated solution from precipitate by filtering. |
Precipitate.
Is not absolutely insoluble in cold alcohol. |
|||||
| Filtrate.
Treated with ammoniated alcohol, gave a light brown precipitate, which was separated by filtration. |
Precipitate.
Suspended in alcohol, decomposed by H2S & filtered. |
|||||
| Filtrate.
Trated with H2S and filtered form the lead sulphide. |
Precipitate.
Suspended in alcohol, and decomposed by H2S; filtered. |
Filtrate.
Yellowish-brown; evaporated to dryness gave a very small amount of a brown red resin; sparingly soluble in ether. |
Precipitate.
Black PbS, containing very little coloring matter. |
|||
| Filtrate.
Evaporated nearly to dryness, gave a red resin, dissolving in cold alcohol, and having the same properties as the crude resin. |
Precipitate.
Black lead sulphide; some little coloring matter; is precipitated with it. |
Filtrate.
A light straw-colored liquid; evaporated to dryness; minute residue. |
Precipitate.
Black PbS, containing little coloring matter. |
|||
By treatment with hot alcohol and cooling, about one-tenth of the resin is precipitated as a dull brown pulverulent substance (x), slightly inclined toward a grey-brown color. The resin (y) that is soluble in cold alcohol, is of a bright red color, extract-like consistence, has a, slight taste, and colors the saliva Their behavior to solvents, etc., was found to be as follows:
| Ether. | Chloroform. | Water. | Carbon Disulphide. | Benzol. | |
| Resin (x) | Spar. soluble, hot or cold. | Spar. soluble, hot or cold. | Sufficient to color only, hot or cold. | Spar. soluble, hot or cold. | Spar sol. hot; less sol. cold. |
| Resin (y) | Very spar. sol. hot or cold. | Soluble, hot or cold. | Scarcely colors, hot or cold. | Soluble hot, spar. soluble cold. | Soluble hot, spar. soluble cold. |
| Gasolin. | Caustic Potassa Sol. | Ammonia. | Hydrochloric Acid. | Incineration. | |
| Resin (x) | Spar. soluble, hot or cold. | Spar. sol. hot; less sol. cold. | Very spar. sol. hot or cold. | Soluble hot, spar. soluble cold. | No ash. |
| Resin (y) | Insoluble, hot or cold. | Sufficient to color, hot or cold. | Spar. sol. hot, insol. cold. | Soluble hot, spar. soluble cold. | Ash. |
The resin (a) was examined for protocatechuic acid as follows: Equal parts of the resin and solid caustic potassa were heated together in a silver dish until completely fused. The dark colored fused mass thus obtained was dissolved in water, the aqueous solution rendered slightly acid by sulphuric acid, and filtered; the yellowish-colored filtrate was then agitated with ether until it ceased to take up any more soluble matter; the ether was then separated and evaporated spontaneously, furnishing a small amount of crystals, which gave with ferric chloride a bright emerald-green color, and on the subsequent addition of a weak solution of potassium hydrate a bright crimson-red color was produced, making it quite conclusive that protocatechuic acid was formed by the above treatment.
Resin (x) gave the same indications for protocatechuic acid when similarly treated.
Examination of Resinous Precipitates in Tincture, etc.—The precipitates formed in the liquid preparations of Sanguinaria on standing were also examined to ascertain whether sanguinarina was carried down with the resinous matter. Messrs. Bullock & Crenshaw, and Wm. R. Warner & Co., very kindly furnished me with sufficient quantities of the precipitate from the tincture and fluid extract, which were examined as follows: The drained precipitate was thoroughly washed with a mixture of alcohol 3 parts, water 1 part, and then boiled with acidulated water (water 15 parts, acetic acid 1 part), filtered, thus separating the resin and giving a dark red nitrate. The nitrate when rendered alkaline with ammonia gave a purplish precipitate; the precipitate was washed and dissolved in ether; hydrochloric acid gas was then passed into the ethereal solution till no further precipitation occurred. A dense bright red precipitate of hydrochlorate of sanguinarina was produced, and a comparatively large quantity for the amount of precipitates employed from both the tincture and fluid extract. Hence the precipitates in liquid preparations of Sanguinaria contain notable quantities of the alkaloid sanguinarina. None of the solvents used or tried would prevent this gradual precipitation; alcohol, however,, proves to be far the best solvent, and not only holds the sanguinarina and resin in solution, but it extracts the resin more completely from the drug.
Properties of the Resin.—In doses of from two to four grains it is a nauseant, reducing the pulse and producing uneasiness in the stomach., In the "Proceedings of the American Pharmaceutical Association" for 1863, page 214, the late Prof. R. P. Thomas gives an exhaustive article on the active principles of Sanguinaria and their therapeutical value. In speaking of the resin he says: "The alkaloid sanguinarina is certainly the most valuable principle existing in bloodroot, but I am persuaded it is not the sole agent, as some trials made with the impure resin show that the latter also possesses nauseant and emetic properties." The examination made on the resin tends to corroborate this statement.
| Examination of Filtrate (b).—To four fluidounces of the filtrate solution of acetate of lead was added, which gave a precipitate of a reddish-purple color. | ||
| Precipitate.
Suspended in water, and decomposed by H2S, filtered, gave a dark red solution; evaporated to extract consistence, was found to consist of a gummy red coloring matter, uncrystallizable. Inert. |
Filtrate.
Treated with subacetate of lead; dense brown precipitate, filtered. |
|
| Precipitate.
Suspended in water, decomposed by H2S and filtered; brown-red solution; evaporated, left gummy extract; proved to be coloring matter. Inert. |
Filtrate.
Light red color; containing the alkaloids, etc. |
|
There seem to be two coloring principles besides the resin and sanguinarina; the one precipitated by normal acetate of lead, the other by basic acetate of lead.
The whole of the filtrate b was then rendered alkaline with ammonia, and the precipitated sanguinarina separated by filtration. The red-brown filtrate was evaporated to an extract and washed with stronger alcohol until this would take up no more (large doses of the residue left after this washing were taken, but proved to be inert). The alcoholic solution was of a dark red color, and contained much glucose, as proven by Trommer's test, the behavior to alcohol and ether and by its sweet taste. The alcohol was evaporated, leaving a sweetish brown-red extract, which was dissolved in water rendered alkaline with potassa, and agitated with ether; the ethereal solution was allowed to evaporate, when it deposited prismatic needle-shaped crystals, colorless, of a very slightly bitter taste, possessing an alkaline reaction, and forming with acids colorless solutions and producing precipitates with solutions of mercurio-potassic iodide and iodine in iodide of potassium. This colorless alkaloid exists in a very minute quantity in the rhizome. With sulphuric acid it gives a beautiful dark purple color, which is not permanent, and changes to a yellowish color after the addition of potassic bichromate. The alkaloid was first isolated by Riegel, in 1845, and its reaction with sulphuric acid was noticed by F. W. Carpenter (see "Amer. Jour. Phar." 1879, p. 172).
The aqueous solution left after washing with ether was found to be inert in large doses. Therefore the medicinal principles are the sanguinarina, resin and perhaps to some extent the second alkaloid. The resin has an effect similar to that produced by the alkaloid, only not in so marked a degree.
The American Journal of Pharmacy, Vol. 53, 1881, was edited by John M. Maisch.

